
 |

| Tracks 1-52 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Discussion
Tracks 1-4
![]() DR LOVE: This woman developed problems after completing treatment
with trastuzumab. What do we know about the time course of
trastuzumab or anthracycline-related cardiotoxicity?
DR LOVE: This woman developed problems after completing treatment
with trastuzumab. What do we know about the time course of
trastuzumab or anthracycline-related cardiotoxicity?
![]() DR DURAND: We know that doxorubicin produces an acute, a subacute and a
long-term cardiotoxicity. One factor that can be difficult to dissect with this
particular patient is whether the decrease in ejection fraction is due to doxorubicin,
one year after exposure, or to trastuzumab.
DR DURAND: We know that doxorubicin produces an acute, a subacute and a
long-term cardiotoxicity. One factor that can be difficult to dissect with this
particular patient is whether the decrease in ejection fraction is due to doxorubicin,
one year after exposure, or to trastuzumab.
![]() DR LOVE: Mark, how typical would it be for a patient to develop congestive
heart failure within a month of completing trastuzumab?
DR LOVE: Mark, how typical would it be for a patient to develop congestive
heart failure within a month of completing trastuzumab?
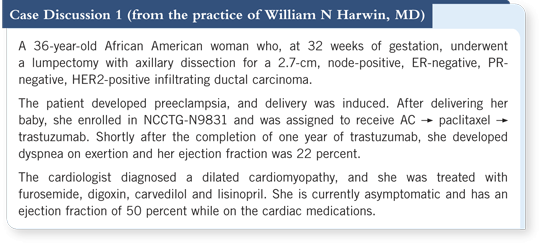
![]() DR PEGRAM: That’s atypical. From NSABP-B-31, we have information about
the time course of trastuzumab-related cardiotoxicity (3.1). It’s important to note
that in that trial about 6.5 percent of the patients never received trastuzumab
because their ejection fraction declined after the four cycles of AC.
DR PEGRAM: That’s atypical. From NSABP-B-31, we have information about
the time course of trastuzumab-related cardiotoxicity (3.1). It’s important to note
that in that trial about 6.5 percent of the patients never received trastuzumab
because their ejection fraction declined after the four cycles of AC.
In the patients who received trastuzumab, a peak seems to have occurred in the incidence of trastuzumab-related cardiotoxicity in the second and third quarter of treatment, which then tapers off again in the fourth quarter (Rastogi 2007; [3.1]). So I would say that this presentation is somewhat atypical for patients with trastuzumab-related cardiotoxicity.
![]() DR LOVE: This patient has been on cardiac medications for a couple of years.
What about stopping the meds?
DR LOVE: This patient has been on cardiac medications for a couple of years.
What about stopping the meds?
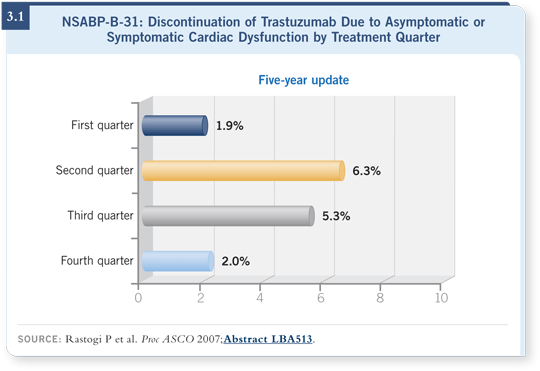
![]() DR DURAND: At our national heart failure meeting, we presented data from
our institution showing that when you withdraw ACE inhibitors and beta-blockers from patients who have anthracycline-related cardiomyopathy, their
ejection fractions decline within 30 days (Lenihan 2003; [3.2]).
DR DURAND: At our national heart failure meeting, we presented data from
our institution showing that when you withdraw ACE inhibitors and beta-blockers from patients who have anthracycline-related cardiomyopathy, their
ejection fractions decline within 30 days (Lenihan 2003; [3.2]).
Tracks 8-10
![]() DR LOVE: Mark, what is your opinion about the cardiac safety of
docetaxel/ carboplatin/trastuzumab (TCH) versus an anthracycline-based
trastuzumab regimen?
DR LOVE: Mark, what is your opinion about the cardiac safety of
docetaxel/ carboplatin/trastuzumab (TCH) versus an anthracycline-based
trastuzumab regimen?
![]() DR PEGRAM: BCIRG 006 compared TCH to AC
DR PEGRAM: BCIRG 006 compared TCH to AC![]() docetaxel/trastuzumab
(TH). TCH was significantly superior to the nontrastuzumab-containing
control arm. Moreover, if you evaluate the outcome associated with TCH
compared to AC
docetaxel/trastuzumab
(TH). TCH was significantly superior to the nontrastuzumab-containing
control arm. Moreover, if you evaluate the outcome associated with TCH
compared to AC![]() TH — even though that study design was not powered to
test noninferiority between those two regimens — you see that the results are
similar (Slamon 2006; [1.2, page 5]).
TH — even though that study design was not powered to
test noninferiority between those two regimens — you see that the results are
similar (Slamon 2006; [1.2, page 5]).
Because more than 1,000 patients are enrolled on each of those arms, it’s likely that any difference between those arms must be trivial. So it’s possible that these nonanthracycline-containing regimens with trastuzumab will be every bit as efficacious as anthracycline-based regimens with trastuzumab.
![]() DR LOVE: If a healthy, 50 to 60-year-old patient without any cardiac risk
factors asks, “What’s the chance that TCH will cause a problem with my
heart?” what do you say?
DR LOVE: If a healthy, 50 to 60-year-old patient without any cardiac risk
factors asks, “What’s the chance that TCH will cause a problem with my
heart?” what do you say?
![]() DR PEGRAM: You have to explain the nuances between clinically significant
cardiac adverse events and asymptomatic declines in ejection fraction
that might prompt one to hold trastuzumab for a month, repeat the echo and
if the echo returns to normal, reintroduce trastuzumab. In terms of clinically
significant cardiotoxicity, it’s 0.4 percent in the TCH arm of BCIRG 006. All
the other asymptomatic declines are largely reversible, and trastuzumab can be
reintroduced during the course of the one year of treatment (Slamon 2006).
DR PEGRAM: You have to explain the nuances between clinically significant
cardiac adverse events and asymptomatic declines in ejection fraction
that might prompt one to hold trastuzumab for a month, repeat the echo and
if the echo returns to normal, reintroduce trastuzumab. In terms of clinically
significant cardiotoxicity, it’s 0.4 percent in the TCH arm of BCIRG 006. All
the other asymptomatic declines are largely reversible, and trastuzumab can be
reintroduced during the course of the one year of treatment (Slamon 2006).
Track 16
![]() DR LOVE: Can you update us on your work with the combination of
trastuzumab and bevacizumab, particularly in terms of cardiac safety?
DR LOVE: Can you update us on your work with the combination of
trastuzumab and bevacizumab, particularly in terms of cardiac safety?
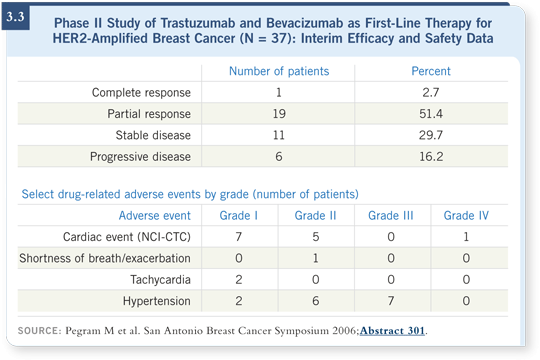
![]() DR PEGRAM: We have completed enrollment of all 50 patients to the Phase II
trastuzumab/bevacizumab trial, which was for patients with HER2-positive
metastatic breast cancer in the first-line setting (Pegram 2006).
DR PEGRAM: We have completed enrollment of all 50 patients to the Phase II
trastuzumab/bevacizumab trial, which was for patients with HER2-positive
metastatic breast cancer in the first-line setting (Pegram 2006).
Early on in the trial, we had a patient who developed Grade IV congestive heart failure, so we conducted an audit of all the cardiac safety adverse events. We basically found a smattering of Grade I and II adverse events, none of which precluded the patients from continuing treatment on the study (3.3).
When you review the Grade I or II cardiac events more closely, you see that all of them were asymptomatic and some were judged to be Grade II because the ejection fraction was one point lower than the normal range.
Tracks 25-30

![]() DR LOVE: Mark, this is an older patient with significant cardiac disease.
What do we know about the safety and efficacy of adjuvant TC? Where
do you think we are heading with nonanthracycline-containing regimens?
DR LOVE: Mark, this is an older patient with significant cardiac disease.
What do we know about the safety and efficacy of adjuvant TC? Where
do you think we are heading with nonanthracycline-containing regimens?
![]() DR PEGRAM: Steve Jones has published data from US Oncology comparing
four cycles of TC to four cycles of AC. The TC regimen compares favorably —
it’s slightly superior to four cycles of AC in terms of efficacy ( Jones 2006; [1.3,
page 6]). Without the anthracycline, one can anticipate an improved cardiac
safety profile.
DR PEGRAM: Steve Jones has published data from US Oncology comparing
four cycles of TC to four cycles of AC. The TC regimen compares favorably —
it’s slightly superior to four cycles of AC in terms of efficacy ( Jones 2006; [1.3,
page 6]). Without the anthracycline, one can anticipate an improved cardiac
safety profile.
US Oncology has launched a new adjuvant trial for patients with HER2-negative, early-stage breast cancer, in which they’ll compare six cycles of TC to six cycles of TAC — the so-called TC-TAC trial. The trial will directly test whether an anthracycline adds any benefit for patients with HER2-negative, early-stage breast cancer. I hope that will put this anthracycline question to rest for patients with HER2-negative disease.
![]() DR LOVE: If a 65-year-old patient without any comorbidities asks, “What’s
the chance that I’m going to have a clinically significant cardiac problem over
the next 20 years from four cycles of AC?” how would you answer?
DR LOVE: If a 65-year-old patient without any comorbidities asks, “What’s
the chance that I’m going to have a clinically significant cardiac problem over
the next 20 years from four cycles of AC?” how would you answer?
![]() DR DURAND: I’d tell her that initially the risk is two to four percent, but as
far out as 20 years, I would tell her at least 10 percent, minimum.
DR DURAND: I’d tell her that initially the risk is two to four percent, but as
far out as 20 years, I would tell her at least 10 percent, minimum.
![]() DR PEGRAM: What struck me about the long-term follow-up data from MD
Anderson (Pinder 2007) is that the incidence of cardiac abnormalities is higher
after four cycles of AC than I would have thought considering long-term
outcomes. The risk is significant, and it continues for a long time.
DR PEGRAM: What struck me about the long-term follow-up data from MD
Anderson (Pinder 2007) is that the incidence of cardiac abnormalities is higher
after four cycles of AC than I would have thought considering long-term
outcomes. The risk is significant, and it continues for a long time.
Tracks 42-44

![]() DR LOVE: Would you consider lapatinib for this patient?
DR LOVE: Would you consider lapatinib for this patient?
![]() DR PEGRAM: Off study, it probably wouldn’t be reimbursed. A new adjuvant
study called ALTTO is being launched for women with HER2-positive,
early-stage disease (3.4).
DR PEGRAM: Off study, it probably wouldn’t be reimbursed. A new adjuvant
study called ALTTO is being launched for women with HER2-positive,
early-stage disease (3.4).
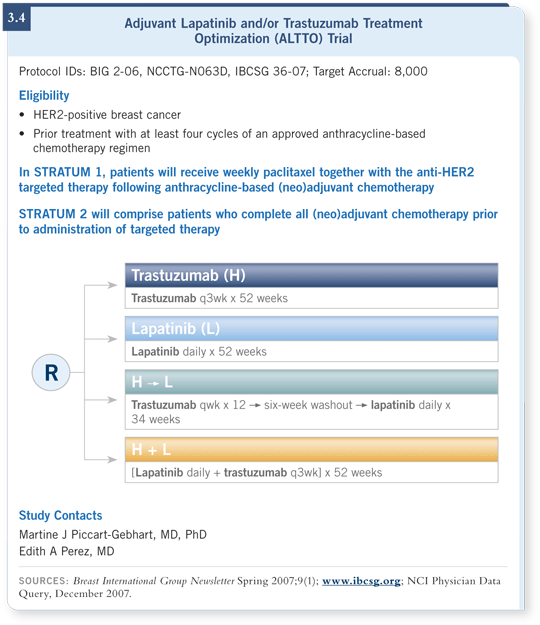
The trial has four arms — patients are randomly assigned to trastuzumab alone, lapatinib alone, the combination or the sequence of trastuzumab and lapatinib. In the meantime, off study, I probably wouldn’t use lapatinib.
A considerable cardiac safety database is emerging for lapatinib-exposed patients. By all accounts, it appears to be associated with fewer cardiac adverse events than trastuzumab administered to similar patients.
Tracks 48-50

![]() DR LOVE: What about the selection of adjuvant chemotherapy for a 79-year-old patient with diabetes, hypertension and hyperlipidemia?
DR LOVE: What about the selection of adjuvant chemotherapy for a 79-year-old patient with diabetes, hypertension and hyperlipidemia?
![]() DR PEGRAM: I wouldn’t have recommended an anthracycline-based regimen
for a 79-year-old. I would have chosen a nonanthracycline and something
that minimizes exposure to chemotherapy. In similar cases, when I’ve chosen
chemotherapy I’ve used four cycles of TC followed by trastuzumab, as in the
HERA trial of sequential chemotherapy and trastuzumab (Smith 2007).
DR PEGRAM: I wouldn’t have recommended an anthracycline-based regimen
for a 79-year-old. I would have chosen a nonanthracycline and something
that minimizes exposure to chemotherapy. In similar cases, when I’ve chosen
chemotherapy I’ve used four cycles of TC followed by trastuzumab, as in the
HERA trial of sequential chemotherapy and trastuzumab (Smith 2007).
![]() DR LOVE: Do you sequence trastuzumab after chemotherapy as a means of
further protection for the heart?
DR LOVE: Do you sequence trastuzumab after chemotherapy as a means of
further protection for the heart?
![]() DR PEGRAM: No, that’s simply the way that data set was generated, and I
follow that scheme. I have no doubt that you could administer TC along with
trastuzumab safely. US Oncology is evaluating TC (docetaxel/cyclophosphamide)
with trastuzumab.
DR PEGRAM: No, that’s simply the way that data set was generated, and I
follow that scheme. I have no doubt that you could administer TC along with
trastuzumab safely. US Oncology is evaluating TC (docetaxel/cyclophosphamide)
with trastuzumab.