
 |
||||||||
![]()

CALGB-40503
Endocrine therapy in combination with anti-VEGF therapy: A randomized, double-blind,
placebo-controlled Phase III trial of endocrine therapy alone or endocrine therapy with
bevacizumab for women with ER/PR-positive advanced breast cancer


CALGB-40503
Endocrine therapy in combination with anti-VEGF therapy: A randomized, double-blind,
placebo-controlled Phase III trial of endocrine therapy alone or endocrine therapy with
bevacizumab for women with ER/PR-positive advanced breast cancer

![]()
“The CALGB 40302 trial is an ongoing study for ER-positive breast cancer, in which patients with metastatic disease receive fulvestrant with or without lapatinib. It is open to patients with both HER2-positive and HER2-negative disease. Our goal is to determine whether dual inhibition of the ER and HER2 pathways is beneficial to either HER2-positive or HER2-negative tumors.
For the moment in HER2-positive metastatic disease, first-line therapy is trastuzumab with chemotherapy. We have compelling survival data for that. Second-line options include ongoing trastuzumab with the chemotherapy of your choice. At some point, one introduces lapatinib. I believe that it’s difficult to be dogmatic about when that moment should be.”
— Harold J Burstein, MD, PhD
Year in Review — A Daylong CME Symposium
Focused on Key Clinical Presentations and
Papers in Oncology: 2007-2008

RIBBON 2 (AVF3693g)
A Phase III, multicenter, randomized, placebo-controlled trial evaluating the efficacy
and safety of bevacizumab in combination with chemotherapy regimens in subjects
with previously treated metastatic breast cancer


CAN-NCIC-MA31
A Phase III randomized trial of taxane-based chemotherapy with lapatinib versus with
trastuzumab for women with HER2-positive metastatic breast cancer


ECOG-E1105
A randomized Phase III trial of first-line chemotherapy and trastuzumab with or without
bevacizumab for patients with HER2-overexpressing metastatic breast cancer


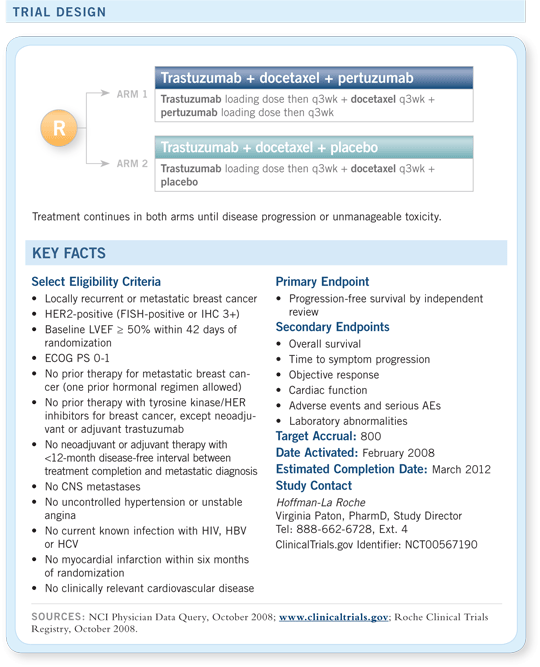
CLEOPATRA
A Phase III, double-blind trial to evaluate the efficacy and safety of trastuzumab and
docetaxel with or without pertuzumab in previously untreated HER2-positive metastatic
breast cancer

VEG108838
A randomized Phase III trial comparing pazopanib and lapatinib versus lapatinib
monotherapy in patients with HER2-overexpressing inflammatory breast cancer

![]()
“Pazopanib (GW786034) is a second-generation multitargeted tyrosine kinase inhibitor against vascular endothelial growth factor receptor-1, -2, and -3, platelet-derived growth factor receptor-alpha, platelet-derived growth factor receptor-beta, and c-kit. Preclinical evaluation has revealed excellent antiangiogenic and antitumor activity, and synergism was observed in combination with chemotherapeutic drugs. Phase I clinical trials have revealed manageable toxicities and desirable pharmacokinetics as well as activity in renal cancer and several other tumors. Ongoing trials are further evaluating pazopanib in a variety of malignancies.”
— Sonpavde G, Hutson TE.
Curr Oncol Rep 2007;9:115-9.

SUN 1064 - A randomized Phase III study of docetaxel in combination with sunitinib versus docetaxel as first-line treatment for patients with advanced breast cancer


A6181094 — A Phase III study of sunitinib in combination with paclitaxel versus bevacizumab with paclitaxel in the first-line advanced disease setting in patients with breast cancer

![]()
“Sunitinib (SU011248) is an oral small molecular tyrosine kinase inhibitor that exhibits potent antiangiogenic and antitumor activity. . . [S]unitinib was rationally designed and chosen for its high bioavailability and its nanomolar-range potency against the antiangiogenic receptor tyrosine kinases (RTKs) — vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR). Sunitinib inhibits other tyrosine kinases including, KIT, FLT3, colony-stimulating factor 1 (CSF-1), and RET, which are involved in a number of malignancies... Studies investigating sunitinib alone in various tumor types and in combination with chemotherapy are ongoing. The clinical benchmarking of this small-molecule inhibitor of members of the split-kinase domain family of RTKs will lead to additional insights regarding the biology, potential biomarkers, and clinical utility of agents that target multiple signaling pathways in tumor, stromal, and endothelial compartments.”
— Chow LQ, Eckhardt SG.
J Clin Oncol 2007;25:884-96.

TRIO-012, CP12-0606
A multicenter, multinational, randomized, double-blind, Phase III study of IMC-1121B (a
fully humanized monoclonal antibody targeting VEGFR-2) and docetaxel versus placebo
and docetaxel in previously untreated patients with HER2-negative, unresectable, locally-recurrent
or metastatic breast cancer

EDITOR'S NOTE
Bail Us Out
Neil Love, MD
CLINICAL TRIALS
Neoadjuvant Therapy
Adjuvant Therapy
Metastatic Disease
Breast Cancer
Clinical Trials Guide:
A CME Audio Series and Activity