
 |
||||||||
![]()

NSABP-B-40
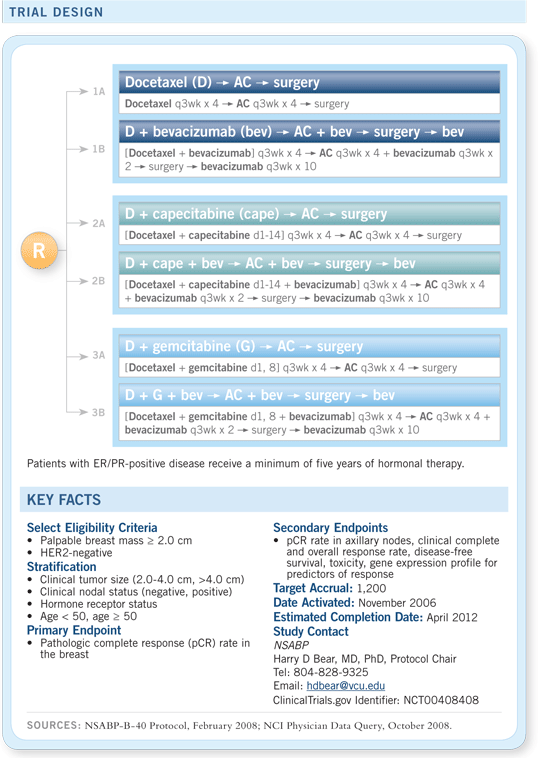
A Phase III randomized trial of six neoadjuvant regimens in patients with palpable and
operable HER2-negative breast cancer

NSABP-B-41
A randomized Phase III trial of neoadjuvant therapy comparing trastuzumab, lapatinib
and the combination administered with weekly paclitaxel after AC


ACOSOG-Z1041
A Phase III trial comparing a neoadjuvant regimen of FEC-75 followed by paclitaxel
and trastuzumab to a neoadjuvant regimen of paclitaxel and trastuzumab followed by
FEC-75 and trastuzumab in patients with HER2-positive operable breast cancer

![]()
“Numerous small phase II studies have shown that adding trastuzumab to preoperative chemotherapy achieves high pCR rates. The MD Anderson group conducted a small, randomized phase II preoperative trial of paclitaxel and FEC with or without trastuzumab in HER-2-overexpressing breast cancer. The pCR rate was 25% in the chemotherapy-only arm versus 67% in the chemotherapy-trastuzumab arm...”
— Gralow J et al. J Clin Oncol 2008;26:814-9.

Neo-ALTTO (Neoadjuvant Lapatinib and/or Trastuzumab Treatment
Optimization) Study
A randomized, multicenter, open-label study of neoadjuvant lapatinib, trastuzumab and their
combination with paclitaxel in women with HER2/ErbB2-positive primary breast cancer

EDITOR'S NOTE
Bail Us Out
Neil Love, MD
CLINICAL TRIALS
Neoadjuvant Therapy
Adjuvant Therapy
Metastatic Disease
Breast Cancer
Clinical Trials Guide:
A CME Audio Series and Activity