
| Tracks 1-6 |
| Track 1 |
Introduction |
| Track 2 |
Background and design of
BCIRG 006 |
| Track 3 |
TOPO II amplification and the
efficacy of anthracycline-based
chemotherapy in BCIRG 006 |
|
| Track 4 |
Schedule and duration of
adjuvant trastuzumab |
| Track 5 |
Clinical implications of adjuvant
trastuzumab trial results |
| Track 6 |
Delayed adjuvant trastuzumab |
|
|
Select Excerpts from the Breast Cancer Update Meet The
Professors Session, held at the 28th Annual San Antonio
Breast Cancer Symposium, December 8-11, 2005
 Track 2-4
Track 2-4
 DR LOVE: Can you summarize the data presented by Dennis Slamon on
the adjuvant trastuzumab trial, BCIRG 006? DR LOVE: Can you summarize the data presented by Dennis Slamon on
the adjuvant trastuzumab trial, BCIRG 006? |
 DR MACKEY: The trial compared four cycles of doxorubicin/cyclophosphamide
followed by four cycles of docetaxel (AC
DR MACKEY: The trial compared four cycles of doxorubicin/cyclophosphamide
followed by four cycles of docetaxel (AC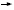 T) — the control arm —
versus four cycles of AC followed by docetaxel plus trastuzumab, which was
administered concurrently with the taxane but then continued for one year
(AC
T) — the control arm —
versus four cycles of AC followed by docetaxel plus trastuzumab, which was
administered concurrently with the taxane but then continued for one year
(AC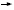 TH), versus an intriguing third arm consisting of docetaxel, carboplatin
and trastuzumab (TCH; [Slamon 2005]).
TH), versus an intriguing third arm consisting of docetaxel, carboplatin
and trastuzumab (TCH; [Slamon 2005]).
On the third arm, all three agents were begun on day one. Six cycles of
chemotherapy were given, and the trastuzumab was continued for one year.
The intent of the trial was to see if the preclinical synergy seen between
docetaxel, carboplatin and trastuzumab would be borne out in the adjuvant
setting and whether we could avoid major problems with cardiotoxicity by
eliminating the anthracycline.
The trial demonstrated that both the AC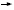 TH arm and the novel arm of
TCH outperformed the control arm, with hazard ratios of 0.49 and 0.61,
respectively. No statistically significant difference appeared between the two
experimental arms.
TH arm and the novel arm of
TCH outperformed the control arm, with hazard ratios of 0.49 and 0.61,
respectively. No statistically significant difference appeared between the two
experimental arms.
In addition, the TCH arm had virtually no cardiotoxicity — only four out of
more than 1,000 patients developed congestive heart failure.
 DR LOVE: Could you comment on the TOPO II data?
DR LOVE: Could you comment on the TOPO II data?
 DR MACKEY: The trial only allowed patients with HER2-positive disease
identified by FISH. All of the tumor blocks were studied in two centers, so we
were able to perform additional molecular analyses.
DR MACKEY: The trial only allowed patients with HER2-positive disease
identified by FISH. All of the tumor blocks were studied in two centers, so we
were able to perform additional molecular analyses.
With HER2 amplification, a small strip of DNA is, by definition, amplified in
this population; however, the HER2 amplicon can include the TOPO II gene,
which is the target for anthracyclines.
Michael Press found that in about a third of patients, the HER2 amplicon
included TOPO II (Press 2005). The really exciting finding was that the
patients who had TOPO II amplification did very well if both targets, TOPO
II and HER2, were hit by using an anthracycline and trastuzumab. In the two
thirds of patients who did not have the amplified TOPO II, TCH performed
just as well as the anthracycline-containing regimen and with no significant
cardiotoxicity.
If we can validate this with additional follow-up, we may be able to select
patients for an optimal adjuvant trastuzumab regimen on the basis of HER2
status in addition to TOPO II amplification by FISH, and these tests can be
done simultaneously. If TOPO II is amplified, we might administer AC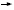 TH;
however, if TOPO II is not amplified, those women could perhaps optimally
be treated with TCH and not exposed to any significant risk of cardiotoxicity.
TH;
however, if TOPO II is not amplified, those women could perhaps optimally
be treated with TCH and not exposed to any significant risk of cardiotoxicity.
 DR LOVE: Is TOPO II ready for “prime time?”
DR LOVE: Is TOPO II ready for “prime time?”
 DR MACKEY: I don’t think so yet, although the data suggest we may finally
have a third predictive assay in breast cancer. We have ER to tell us whether
we should be using hormonal therapy, and we have HER2 to tell us whether
we need trastuzumab. My prediction is that TOPO II amplification will
become a validated predictive assay for benefit from anthracyclines, but we
haven’t proved that yet.
DR MACKEY: I don’t think so yet, although the data suggest we may finally
have a third predictive assay in breast cancer. We have ER to tell us whether
we should be using hormonal therapy, and we have HER2 to tell us whether
we need trastuzumab. My prediction is that TOPO II amplification will
become a validated predictive assay for benefit from anthracyclines, but we
haven’t proved that yet.
Select publications

