
| Tracks 1-12 |
| Track 1 |
Introduction |
| Track 2 |
Selection of adjuvant therapy for
women with breast cancer |
| Track 3 |
TOPO II amplification and the
efficacy of anthracycline-based
chemotherapy in BCIRG 006 |
| Track 4 |
Clinical use of adjuvant trastuzumab |
| Track 5 |
Management of HER2-positive,
node-negative disease |
| Track 6 |
Combining adjuvant trastuzumab
with dose-dense chemotherapy |
|
| Track 7 |
Delayed adjuvant trastuzumab |
| Track 8 |
Future directions for clinical
research in HER2-positive disease |
| Track 9 |
Clinical use of bevacizumab for
patients with metastatic breast
cancer |
| Track 10 |
Selection of first-line chemotherapy
for patients with metastatic disease |
| Track 11 |
Selection of up-front adjuvant
hormonal therapy for postmenopausal
women |
| Track 12 |
Impact of progesterone receptor
status on selection of adjuvant
hormonal therapy |
|
|
Select Excerpts from the Interview
 Track 2
Track 2
 DR LOVE: What do you consider the optimal adjuvant chemotherapy
regimen for a patient with a node-positive tumor? DR LOVE: What do you consider the optimal adjuvant chemotherapy
regimen for a patient with a node-positive tumor? |
 DR PICCART-GEBHART: I believe that in the not-too-distant future, we will
approach the choice of chemotherapy completely differently. We used to think
according to risk, dividing the choice of chemotherapy regimens into the
most appropriate for patients with node-positive versus node-negative disease
(Piccart-Gebhart 2005a).
DR PICCART-GEBHART: I believe that in the not-too-distant future, we will
approach the choice of chemotherapy completely differently. We used to think
according to risk, dividing the choice of chemotherapy regimens into the
most appropriate for patients with node-positive versus node-negative disease
(Piccart-Gebhart 2005a).
We are going to move away from that, because we are entering an era in
cancer medicine with the development of superb tools to predict which tumors
respond to which drug.
We are not there yet, but this is going fast. The technologies are exploding.
If we, the clinicians, are smart enough to design the right studies to validate
these technologies quickly, it’s going to change the picture.
Instead of our habit of thinking that six positive nodes means dose-dense
chemotherapy, we should look at the profile of the tumor first. And after that
we can look at the nodes, because the number of nodes is related to risk.
I certainly would never administer dose-dense chemotherapy to a patient with
a Grade I, highly endocrine-responsive tumor with maximum receptors and
a very low proliferation index. On the contrary, if I see a young patient with
negative nodes but an aggressive tumor with absolutely no endocrine receptors
whatsoever, no HER2 and very high proliferation, I would be tempted to use
dose-dense therapy.
 DR LOVE: Does that same concept apply, for example, to using trastuzumab
for patients with lower-risk, node-negative disease?
DR LOVE: Does that same concept apply, for example, to using trastuzumab
for patients with lower-risk, node-negative disease?
 DR PICCART-GEBHART: This reinforces what I was saying. When a test tells
you the tumor will have a good chance of responding to a targeted drug, you
more readily use this drug in node-negative patients.
DR PICCART-GEBHART: This reinforces what I was saying. When a test tells
you the tumor will have a good chance of responding to a targeted drug, you
more readily use this drug in node-negative patients.
In the adjuvant trastuzumab studies, especially the HERA trial, which had
30 percent of node-negative patients, we saw a substantial degree of benefit
from trastuzumab (HERA 2005; Piccart-Gebhart 2005b). The relative risk
reduction was the same as among patients with node-positive disease; it was a
substantial gain.
As we become smarter about identifying the drugs that work in particular
tumors, we will still consider risk in the clinical decision-making process,
but it will come second. Risk will be used to evaluate the tradeoffs between
efficacy and toxicity, but it will no longer be the first consideration in treatment
decision-making.
 Track 3
Track 3
 DR LOVE: How do you view the results of the BCIRG adjuvant
trastuzumab trial? DR LOVE: How do you view the results of the BCIRG adjuvant
trastuzumab trial? |
 DR PICCART-GEBHART: The BCIRG 006 study is truly an interesting study.
It is original in the sense that it’s the only adjuvant trastuzumab trial that has
at least one arm without an anthracycline, the TCH arm. I view the results
of this arm as extremely positive. Several of my colleagues were disappointed,
because everybody was expecting TCH to markedly surpass all the others in
view of some interesting preclinical data (Slamon 2005).
DR PICCART-GEBHART: The BCIRG 006 study is truly an interesting study.
It is original in the sense that it’s the only adjuvant trastuzumab trial that has
at least one arm without an anthracycline, the TCH arm. I view the results
of this arm as extremely positive. Several of my colleagues were disappointed,
because everybody was expecting TCH to markedly surpass all the others in
view of some interesting preclinical data (Slamon 2005).
My interpretation of the results is that the TCH arm is almost as good as the
traditional AC followed by a taxane and trastuzumab, in terms of efficacy, and
it clearly has the advantage of a much reduced risk of cardiac toxicity. I view
this regimen as a good option for a woman at very high risk, for example,
with many positive nodes, about whom I am very concerned about the cardiac
toxicity risk.
 DR LOVE: Can you summarize your take on the TOPO II data (Press 2005)?
Do you think that right now it would be reasonable to utilize this test as a
guide in clinical practice?
DR LOVE: Can you summarize your take on the TOPO II data (Press 2005)?
Do you think that right now it would be reasonable to utilize this test as a
guide in clinical practice?
 DR PICCART-GEBHART: You do some things in medicine before they are
completely evidence based. In this case, it is not the first observation. Many
others have been recorded, and the data already look pretty solid. So although
you certainly cannot write that in a textbook or in guidelines, I will be
tempted to use it for my patients.
DR PICCART-GEBHART: You do some things in medicine before they are
completely evidence based. In this case, it is not the first observation. Many
others have been recorded, and the data already look pretty solid. So although
you certainly cannot write that in a textbook or in guidelines, I will be
tempted to use it for my patients.
 Track 5
Track 5
 DR LOVE: How do you approach patients right now with small HER2-
positive tumors and negative nodes? DR LOVE: How do you approach patients right now with small HER2-
positive tumors and negative nodes? |
 DR PICCART-GEBHART: Women were allowed to enter the HERA trial if the
tumor size was greater than one centimeter. This was the only criterion. We
didn’t require other aggressive features — it was purely based on pathological
size. Of course, now the problem we have is with young women coming to us
with eight-millimeter tumors and negative nodes. Usually, when you look at
the pathology, you see other features of aggressiveness — for example, a high
proliferation rate, Grade III tumors and so on.
DR PICCART-GEBHART: Women were allowed to enter the HERA trial if the
tumor size was greater than one centimeter. This was the only criterion. We
didn’t require other aggressive features — it was purely based on pathological
size. Of course, now the problem we have is with young women coming to us
with eight-millimeter tumors and negative nodes. Usually, when you look at
the pathology, you see other features of aggressiveness — for example, a high
proliferation rate, Grade III tumors and so on.
Personally, I don’t see why these women would not derive a substantial
benefit from trastuzumab. Provided these women are well informed about
cardiotoxicity risk, of course, we are discussing with them the possibility
of trastuzumab.
 Track 7
Track 7
 DR LOVE: What are your thoughts about the issue of delayed trastuzumab
for the patient who was diagnosed one, two or five years ago with significant
residual risk? DR LOVE: What are your thoughts about the issue of delayed trastuzumab
for the patient who was diagnosed one, two or five years ago with significant
residual risk? |
 DR PICCART-GEBHART: That’s a very difficult question. As we continue to
follow the women in the control arms of the adjuvant trastuzumab trials, we
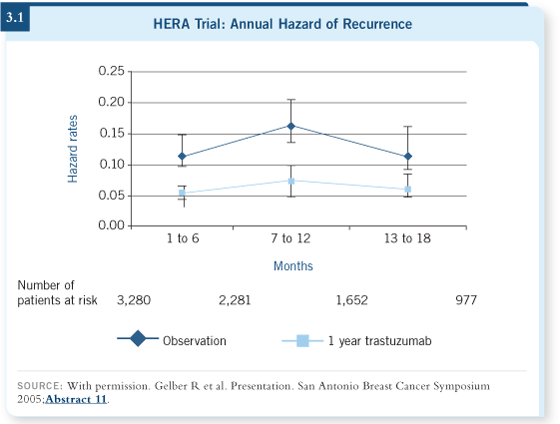
will better understand the hazard rates over time. It’s clear from the data we
collected in HERA that women are at very high risk in the first two years
— they have a high risk of experiencing a relapse every year (HERA 2005;
Piccart-Gebhart 2005b; [3.1]). So up to two years, if a woman wants to start
the drug, it makes sense to do it. Beyond two years, I have no idea.
DR PICCART-GEBHART: That’s a very difficult question. As we continue to
follow the women in the control arms of the adjuvant trastuzumab trials, we
will better understand the hazard rates over time. It’s clear from the data we
collected in HERA that women are at very high risk in the first two years
— they have a high risk of experiencing a relapse every year (HERA 2005;
Piccart-Gebhart 2005b; [3.1]). So up to two years, if a woman wants to start
the drug, it makes sense to do it. Beyond two years, I have no idea.
Our French colleagues demonstrated this with tamoxifen, another targeted
agent. Dr Goss also presented fascinating data at the San Antonio meeting
with delayed introduction of letrozole. So you start wondering whether a
smart targeted drug will still provide benefit when you introduce it somewhat
later. It would be interesting to design a trial looking at that (Goss 2005 a, b).
 Track 9
Track 9
 DR LOVE: Where do you see bevacizumab fitting in with clinical
management of metastatic breast cancer? DR LOVE: Where do you see bevacizumab fitting in with clinical
management of metastatic breast cancer? |
 DR PICCART-GEBHART: That’s a difficult question, and I don’t think I have an
answer. I am embarrassed by the fact that it’s not possible to identify the subset
of patients that really benefits from the drug. To me, using such an expensive
agent for metastatic disease without the possibility of targeting the drug is a
little bit problematic.
DR PICCART-GEBHART: That’s a difficult question, and I don’t think I have an
answer. I am embarrassed by the fact that it’s not possible to identify the subset
of patients that really benefits from the drug. To me, using such an expensive
agent for metastatic disease without the possibility of targeting the drug is a
little bit problematic.
Putting cost aside, I would use clinical judgment and I would probably be
comfortable with the use of the drug for very aggressive, rapidly proliferating
tumors with visceral metastasis for which, clearly, endocrine therapy cannot be
beneficial.
I have no personal experience with the drug, because we have not had access
to it in Europe, with the exception of a few investigators involved in Phase
I/Phase II trials. It is also not reimbursed. So not having experience makes it
difficult to answer.
But in my experience, sometimes women are exposed to endocrine treatments
in sequence for prolonged periods of time. The day they exhaust all these
possibilities, they are endocrine resistant, and you believe that chemotherapy
is going to work, because they haven’t seen it. My impression is that it doesn’t
work that well in that setting.
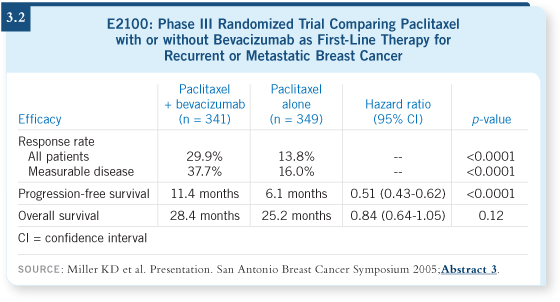
Based on that experience and given that there is this very well-conducted,
highly positive randomized trial, I would have few problems prescribing
bevacizumab combined with a taxane. If I were to use this drug today,
I would use it with weekly paclitaxel as it was given in the ECOG-E2100 trial
(Miller 2005; [3.2]).
 Track 10
Track 10
 DR LOVE: In general, what tends to be your first-line chemotherapy? DR LOVE: In general, what tends to be your first-line chemotherapy? |
 DR PICCART-GEBHART: The majority of first-line chemotherapy in Europe is
taxane-based, because we give a lot of anthracyclines in the adjuvant setting.
Many people use taxanes as soon as there is visceral disease — either docetaxel
or paclitaxel.
DR PICCART-GEBHART: The majority of first-line chemotherapy in Europe is
taxane-based, because we give a lot of anthracyclines in the adjuvant setting.
Many people use taxanes as soon as there is visceral disease — either docetaxel
or paclitaxel.
For patients who received adjuvant taxanes, we tend to use them again if there
is a long treatment-free interval.
 DR LOVE: What about if there isn’t a long treatment-free interval or if the
patient progressed on a taxane?
DR LOVE: What about if there isn’t a long treatment-free interval or if the
patient progressed on a taxane?
 DR PICCART-GEBHART: Then it would depend a little bit on the amount of
anthracycline they had, and whether we can possibly use a liposomal anthracycline.
Capecitabine, clearly, is among our choices. Vinorelbine may be another
option.
DR PICCART-GEBHART: Then it would depend a little bit on the amount of
anthracycline they had, and whether we can possibly use a liposomal anthracycline.
Capecitabine, clearly, is among our choices. Vinorelbine may be another
option.
 DR LOVE: What has been your experience with capecitabine in the metastatic
setting?
DR LOVE: What has been your experience with capecitabine in the metastatic
setting?
 DR PICCART-GEBHART: For some patients this can really be a fabulous treatment.
I have a few patients who have been on capecitabine for more than
two years with very good stabilization of the disease, manageable toxicities
and good quality of life. When the drug works, it can be really tremendously
useful.
DR PICCART-GEBHART: For some patients this can really be a fabulous treatment.
I have a few patients who have been on capecitabine for more than
two years with very good stabilization of the disease, manageable toxicities
and good quality of life. When the drug works, it can be really tremendously
useful.
 DR LOVE: How do you approach dosing?
DR LOVE: How do you approach dosing?
 DR PICCART-GEBHART: I usually start at 2,000 mg/m2. In a very frail patient
I might even start with 1,800 mg/m2. I never start with 2,500 mg/m2 — I
reduce the dose.
DR PICCART-GEBHART: I usually start at 2,000 mg/m2. In a very frail patient
I might even start with 1,800 mg/m2. I never start with 2,500 mg/m2 — I
reduce the dose.
 Track 11
Track 11
 DR LOVE: What is your current take on adjuvant endocrine therapy,
particularly for postmenopausal patients in a clinical setting? DR LOVE: What is your current take on adjuvant endocrine therapy,
particularly for postmenopausal patients in a clinical setting? |
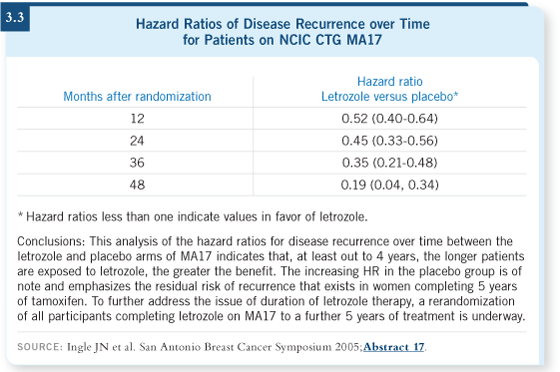
 DR PICCART-GEBHART: I have been very impressed by the results of MA17
(Goss 2003, 2005a; Ingle 2005; [3.3]). To me, this is an indication that
hormone receptor-positive breast cancer is extremely difficult to cure — these
women are at risk of relapse five, seven, 10, even 12 years after diagnosis. On
the other hand, we clearly have an increasing number of very active endocrine
agents.
DR PICCART-GEBHART: I have been very impressed by the results of MA17
(Goss 2003, 2005a; Ingle 2005; [3.3]). To me, this is an indication that
hormone receptor-positive breast cancer is extremely difficult to cure — these
women are at risk of relapse five, seven, 10, even 12 years after diagnosis. On
the other hand, we clearly have an increasing number of very active endocrine
agents.
I am biased. I believe that the optimal therapy for these women in the future
will be a very smart sequence of endocrine agents, covering at least 10 years.
Because of this bias, I don’t like the idea of giving an aromatase inhibitor to
everybody up front, because I don’t know what to give after an AI, and I don’t
think an aromatase inhibitor will cure all these women. In addition, some
patients will develop resistance to the drug.
In view of that, I tend to look at the profile of the tumor. If I’m dealing with
a highly endocrine-responsive tumor with little worry about early relapse on
therapy — a situation in which both ER and PR are very high, the proliferation
genes are very low, the tumor is Grade I, and there is no HER2 overexpression
— I believe there is a very low risk that the patient will relapse if you
put her on tamoxifen for two years.
 DR LOVE: What if the patient has node-positive disease? Do you believe that
in the first two or three years there is an excess risk of relapse in those patients
on tamoxifen?
DR LOVE: What if the patient has node-positive disease? Do you believe that
in the first two or three years there is an excess risk of relapse in those patients
on tamoxifen?
 DR PICCART-GEBHART: It might be that you will have a few early relapses, but
on the other hand, given that the sequencing strategy is so effective and allows
you to go for a seven- or eight-year treatment period, you might recover these
losses later. The sequencing strategy might be more effective in the long term.
DR PICCART-GEBHART: It might be that you will have a few early relapses, but
on the other hand, given that the sequencing strategy is so effective and allows
you to go for a seven- or eight-year treatment period, you might recover these
losses later. The sequencing strategy might be more effective in the long term.
Of course, this is pure speculation and nobody knows. But I don’t think it’s so
simple. An aromatase inhibitor for everybody up front is also expensive and
cannot be afforded in many parts of the world. Certainly, this might not be
the best strategy for everyone.
 DR LOVE: How do you factor endometrial cancer and deep vein thrombosis
versus potential effects on bone into your decision?
DR LOVE: How do you factor endometrial cancer and deep vein thrombosis
versus potential effects on bone into your decision?
 DR PICCART-GEBHART: It is very important. Clearly, before making a decision
about adjuvant endocrine treatment, I do an in-depth evaluation of lipids,
cardiovascular status and bone health.
DR PICCART-GEBHART: It is very important. Clearly, before making a decision
about adjuvant endocrine treatment, I do an in-depth evaluation of lipids,
cardiovascular status and bone health.
All of these considerations need to be factored into the decision-making
process.
 DR LOVE: Putting aside the cost issue, what would you recommend for a 70-
year-old woman with an ER/PR-positive tumor with two positive nodes?
DR LOVE: Putting aside the cost issue, what would you recommend for a 70-
year-old woman with an ER/PR-positive tumor with two positive nodes?
 DR PICCART-GEBHART: This is clearly a woman for whom I am going to
investigate the family history, look at lipid levels, ask for bone densitometry,
ask whether she already has arthralgia and make sure that she has no history of
deep venous thrombosis and embolism in the family.
DR PICCART-GEBHART: This is clearly a woman for whom I am going to
investigate the family history, look at lipid levels, ask for bone densitometry,
ask whether she already has arthralgia and make sure that she has no history of
deep venous thrombosis and embolism in the family.
If she’s in very good health, very active, very fit, and I don’t have to worry too
much about bone and about cardiovascular disease, I would start with tamoxifen
and go after that to an aromatase inhibitor, despite her two positive nodes.
And this woman can still have a long life expectancy.
Starting with four positive nodes, I am more reluctant to go for tamoxifen.
But a patient with one to three positive nodes is still in an intermediate risk
group in the absence of HER2 overexpression.
 DR LOVE: If we were able to conduct an international Patterns of Care study,
I would expect to see a big difference in terms of US versus non-US physicians
in how they answer that case.
DR LOVE: If we were able to conduct an international Patterns of Care study,
I would expect to see a big difference in terms of US versus non-US physicians
in how they answer that case.
 DR PICCART-GEBHART: I agree. Although even in the US I see the controversy.
I know colleagues who are very much in favor of the sequencing
strategy in selected patients and others who are really in favor of an aromatase
inhibitor. Some have killed tamoxifen and don’t use it anymore.
DR PICCART-GEBHART: I agree. Although even in the US I see the controversy.
I know colleagues who are very much in favor of the sequencing
strategy in selected patients and others who are really in favor of an aromatase
inhibitor. Some have killed tamoxifen and don’t use it anymore.
Select publications

