
| Tracks 1-20 |
| Track 1 |
Introduction |
| Track 2 |
Role of hormone-receptor
status in decision-making about
adjuvant therapy |
| Track 3 |
Selection of a chemotherapeutic
regimen in the adjuvant setting |
| Track 4 |
Docetaxel/cyclophosphamide
versus doxorubicin/cyclophosphamide
as adjuvant therapy |
| Track 5 |
Selection of up-front adjuvant
hormonal therapy |
| Track 6 |
Tolerability of aromatase
inhibitors versus tamoxifen |
| Track 7 |
Role of the bisphosphonates in
the prevention of metastases |
| Track 8 |
Potential use of aromatase
inhibitors in the prevention setting |
| Track 9 |
Duration of adjuvant therapy with
an aromatase inhibitor |
| Track 10 |
Oxford Overview: Disease-free
survival for patients with
ER-positive and ER-negative
untreated breast cancer |
|
| Track 11 |
Trend toward survival benefit
in the trials of adjuvant
aromatase inhibitors |
| Track 12 |
Benefit of delayed letrozole
observed in MA17 |
| Track 13 |
Cardiac toxicity associated with
aromatase inhibitors |
| Track 14 |
Women’s Intervention Nutrition
Study (WINS): Impact of
reduction in dietary fat intake
on recurrence |
| Track 15 |
Influence of TOPO II amplification
on the efficacy of anthracycline-based
chemotherapy in
BCIRG 006 |
| Track 16 |
Clinical use of adjuvant
trastuzumab |
| Track 17 |
Combining trastuzumab with
epirubicin |
| Track 18 |
Adjuvant trastuzumab in patients
with HER2-positive, node-negative
breast cancer |
| Track 19 |
Delayed adjuvant trastuzumab |
| Track 20 |
Incidence of brain metastases in
patients receiving trastuzumab |
|
|
Select Excerpts from the Interview
 Track 5-6
Track 5-6
 DR LOVE: Dr Martine Piccart-Gebhart, in her presentation at the San
Antonio Breast Cancer Symposium, suggested that some postmenopausal
patients are better served with tamoxifen, at least initially (Piccart-
Gebhart 2005). What are your thoughts on that? DR LOVE: Dr Martine Piccart-Gebhart, in her presentation at the San
Antonio Breast Cancer Symposium, suggested that some postmenopausal
patients are better served with tamoxifen, at least initially (Piccart-
Gebhart 2005). What are your thoughts on that? |
 DR PRITCHARD: That point of view relates to the fact that the hazard ratios
in the switching trials looked somewhat better than those in the trials that
compared an aromatase inhibitor to tamoxifen from the beginning. Also,
many of us thought that priming with tamoxifen prior to treatment with an
aromatase inhibitor might somehow be advantageous.
DR PRITCHARD: That point of view relates to the fact that the hazard ratios
in the switching trials looked somewhat better than those in the trials that
compared an aromatase inhibitor to tamoxifen from the beginning. Also,
many of us thought that priming with tamoxifen prior to treatment with an
aromatase inhibitor might somehow be advantageous.
However, when you consider randomized studies of up-front AIs in which
disease recurs more in patients on tamoxifen than in those on the aromatase
inhibitor in the first two years, it’s difficult to suggest that you should begin
with tamoxifen (Howell 2005; Thürlimann 2005).
Until somebody shows in a randomized fashion that patients who begin with
adjuvant tamoxifen are doing better at the end of five years than the patients
who use an aromatase inhibitor initially, I will discuss with virtually all my
patients the idea of beginning therapy with an aromatase inhibitor.
 DR LOVE: How do you find tamoxifen and aromatase inhibitors compare
with regard to toxicities?
DR LOVE: How do you find tamoxifen and aromatase inhibitors compare
with regard to toxicities?
 DR PRITCHARD: The aromatase inhibitors probably have a better toxicity
profile. The side effects of tamoxifen are different, and while some are not of
concern for everyone, such as endometrial cancer for women who have had
a hysterectomy, we still have to be concerned about deep vein thrombosis,
which is a serious complication.
DR PRITCHARD: The aromatase inhibitors probably have a better toxicity
profile. The side effects of tamoxifen are different, and while some are not of
concern for everyone, such as endometrial cancer for women who have had
a hysterectomy, we still have to be concerned about deep vein thrombosis,
which is a serious complication.
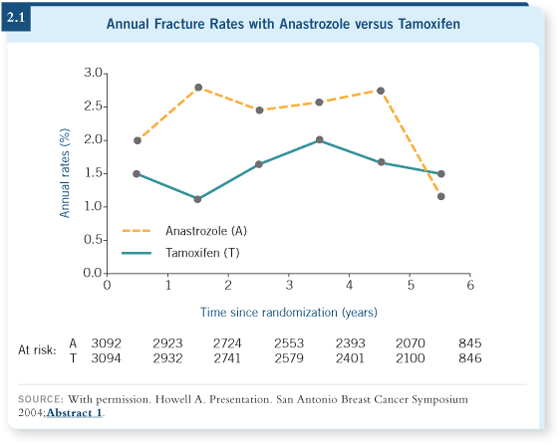
With the aromatase inhibitors, we’re seeing more osteoporosis (2.1). In the
MA17 data, Goss showed more fractures and osteoporosis in the patients on
the placebo arm of the original trial who crossed over to letrozole in the last
two years after unblinding compared to those who did not (Goss 2005a).
I think we will see some long-term complications from this unless these
patients are properly treated for their osteoporosis. We have to consider how
well we are prepared to either treat our patients or collaborate with primary
caregivers to prevent osteoporosis, which I think is not well managed in the
general population.
In Canada, increasingly, we find patients are not always screened or treated
according to the guidelines. In addition, patients may not have a family
doctor, or they don’t follow their physician’s recommendations, so it’s a bit out
of our control.
 Track 8
Track 8
 DR LOVE: A number of ongoing trials are evaluating the efficacy of
aromatase inhibitors in the prevention setting and in the treatment of
DCIS. When the tamoxifen prevention data were released, they generated
a lot of excitement, and yet people don’t use it very much in that setting.
What do you think is the potential of aromatase inhibitors in prevention? DR LOVE: A number of ongoing trials are evaluating the efficacy of
aromatase inhibitors in the prevention setting and in the treatment of
DCIS. When the tamoxifen prevention data were released, they generated
a lot of excitement, and yet people don’t use it very much in that setting.
What do you think is the potential of aromatase inhibitors in prevention? |
 DR PRITCHARD: People have opted not to take tamoxifen for prevention,
which is actually quite startling. It seems women intuitively don’t want to take a pill to prevent something, and I’m not sure why that is, but I think we will
see some of the same problems with the aromatase inhibitors.
DR PRITCHARD: People have opted not to take tamoxifen for prevention,
which is actually quite startling. It seems women intuitively don’t want to take a pill to prevent something, and I’m not sure why that is, but I think we will
see some of the same problems with the aromatase inhibitors.
People in general like to believe that maybe there’s a lifestyle intervention that
would be equally or more effective than taking a drug. If one exists for breast
cancer, I don’t think we know what it is. We are learning that exercise and
keeping one’s weight down may be important, and obviously these measures
are good for patients in a number of ways.
I think the BRCA gene carriers are the only women who have really
embraced the idea of chemoprevention. I suspect that will be true with the
aromatase inhibitors as well, particularly if osteoporosis is an issue.
 DR LOVE: How do you find that aromatase inhibitors compare with
tamoxifen in terms of tolerability?
DR LOVE: How do you find that aromatase inhibitors compare with
tamoxifen in terms of tolerability?
 DR PRITCHARD: The hot flashes with tamoxifen are real. They show up in
every placebo study, whereas weight gain and a number of other complaints
do not. I believe patients similarly experience hot f lashes on aromatase inhibitors,
and while these may not bother some patients, they can be deal breakers
for others.
DR PRITCHARD: The hot flashes with tamoxifen are real. They show up in
every placebo study, whereas weight gain and a number of other complaints
do not. I believe patients similarly experience hot f lashes on aromatase inhibitors,
and while these may not bother some patients, they can be deal breakers
for others.
If we’re going to treat patients with an aromatase inhibitor long term in the
adjuvant or prevention setting, we will probably use an aromatase inhibitor
plus a bisphosphonate, which may double the potential for toxicity but may
double the benefit as well.
 Track 8
Track 8
 DR LOVE: In your practice, what has been your patients’ experience with
arthralgias and AIs? DR LOVE: In your practice, what has been your patients’ experience with
arthralgias and AIs? |
 DR PRITCHARD: The absolute difference between the arthralgias in patients
on aromatase inhibitors versus tamoxifen is about five percent. I believe the
aches and pains that patients experience with aromatase inhibitors are real, but
it’s such a peculiar phenomenon.
DR PRITCHARD: The absolute difference between the arthralgias in patients
on aromatase inhibitors versus tamoxifen is about five percent. I believe the
aches and pains that patients experience with aromatase inhibitors are real, but
it’s such a peculiar phenomenon.
Some of these women become miserable, and when you discontinue the drug,
for many, the symptoms disappear. However, I’ve had some patients that I’ve
put back on tamoxifen, and they still have the aches and pains.
I guess this side effect is related to the lowering of estrogen. Aches and pains
are reported as a menopausal symptom and are generally regarded as not all
that common or serious, but maybe we don’t always listen to what women tell
us about their menopausal symptoms.
 Track 10
Track 10
 DR LOVE: Putting reimbursement issues aside, how do you manage
women who have received five years of an aromatase inhibitor or who
switched at two or three years and get to the five-year point? DR LOVE: Putting reimbursement issues aside, how do you manage
women who have received five years of an aromatase inhibitor or who
switched at two or three years and get to the five-year point? |
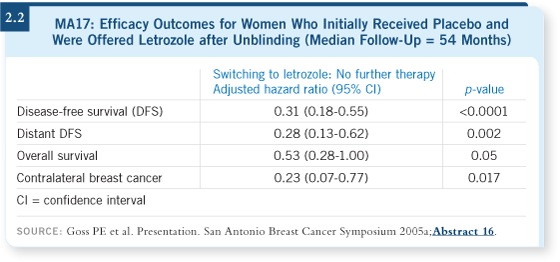
 DR PRITCHARD: We’re just starting to see these women now, and we don’t
know what to do. I told the last patient I saw to continue her aromatase
inhibitor and come back in six months because we would have a clinical trial
for her. Both Jim Ingle and Paul Goss have presented data from the MA17 trial
that suggest year upon year, letrozole continues to add benefit (Ingle 2005;
Goss 2005a; [2.2]).
DR PRITCHARD: We’re just starting to see these women now, and we don’t
know what to do. I told the last patient I saw to continue her aromatase
inhibitor and come back in six months because we would have a clinical trial
for her. Both Jim Ingle and Paul Goss have presented data from the MA17 trial
that suggest year upon year, letrozole continues to add benefit (Ingle 2005;
Goss 2005a; [2.2]).
However, until we see randomized studies, we’re not going to know the best
way to manage these cases. I think it’s great that the NSABP is launching a
study to evaluate patients who have had five years of any aromatase inhibitor or two or three years of tamoxifen followed by an aromatase inhibitor. These
patients will then be randomly assigned to an additional five years of an
aromatase inhibitor or not.
 Track 10
Track 10
 DR LOVE: Can you talk about the data that were presented at the Oxford
Trialists’ meeting in September 2005, regarding disease-free survival
curves for ER-positive and ER-negative breast cancer? DR LOVE: Can you talk about the data that were presented at the Oxford
Trialists’ meeting in September 2005, regarding disease-free survival
curves for ER-positive and ER-negative breast cancer? |
 DR PRITCHARD: The most interesting piece of data I saw was a curve showing
that disease in untreated patients with ER-negative disease recurs quickly in
the first few years, but then their curves level out much more than patients
with ER-positive disease.
DR PRITCHARD: The most interesting piece of data I saw was a curve showing
that disease in untreated patients with ER-negative disease recurs quickly in
the first few years, but then their curves level out much more than patients
with ER-positive disease.
On the other hand, untreated patients with ER-positive disease do much
better in the first five years, and they’re still ahead in the next five years.
However, at approximately 10 years, the disease-free survival curves for ER-positive
and ER-negative disease cross over each other, and at 15 years, the
survival curves are crossing.
 DR LOVE: So the untreated patients with ER-positive disease have a higher
delayed relapse rate than those with ER-negative disease?
DR LOVE: So the untreated patients with ER-positive disease have a higher
delayed relapse rate than those with ER-negative disease?
 DR PRITCHARD: Yes. It’s slower and steadier, but they keep recurring. It
makes sense that we’re now seeing that treatment after five years can be very
helpful, because these patients have an ongoing risk. We haven’t all appreciated
this very well until the last few years. I believe that the Saphner paper showed
this ongoing risk, and the Oxford Overview data have shown this before as
well (Saphner 1996).
DR PRITCHARD: Yes. It’s slower and steadier, but they keep recurring. It
makes sense that we’re now seeing that treatment after five years can be very
helpful, because these patients have an ongoing risk. We haven’t all appreciated
this very well until the last few years. I believe that the Saphner paper showed
this ongoing risk, and the Oxford Overview data have shown this before as
well (Saphner 1996).
We all think of ER-positive disease as having a better natural history, but the
fact is that by 10 years, more of the patients with ER-positive disease have
recurred than the ER-negative group, both untreated. It’s shocking because we
thought we could treat these patients with tamoxifen and after that they would
do well and we would not have to worry about them, but they continue on
having recurrences.
So I think adding additional treatment with an aromatase inhibitor or
certainly evaluating these patients in clinical trials is important.
 Track 11
Track 11
 DR LOVE: Do you think the adjuvant aromatase inhibitor trials will
eventually show a mortality benefit? DR LOVE: Do you think the adjuvant aromatase inhibitor trials will
eventually show a mortality benefit? |
 DR PRITCHARD: I believe that if these trials had gone on long enough, we
definitely would have seen a mortality benefit. In the MA17 trial, when the
trial was stopped, approximately 70 percent of the patients crossed over to
letrozole. We’re still seeing a significant overall survival benefit in patients with node-positive disease who were randomly assigned to letrozole. Had the
original trial continued another six or 12 months, we would have seen that
much more clearly; however, now as patients cross over and receive the benefit
but receive it later, that mortality benefit may be muted.
DR PRITCHARD: I believe that if these trials had gone on long enough, we
definitely would have seen a mortality benefit. In the MA17 trial, when the
trial was stopped, approximately 70 percent of the patients crossed over to
letrozole. We’re still seeing a significant overall survival benefit in patients with node-positive disease who were randomly assigned to letrozole. Had the
original trial continued another six or 12 months, we would have seen that
much more clearly; however, now as patients cross over and receive the benefit
but receive it later, that mortality benefit may be muted.
 DR LOVE: Can you talk about the meta-analysis that was presented at the San
Antonio Breast Cancer Symposium on the mortality data from the aromatase
inhibitor trials?
DR LOVE: Can you talk about the meta-analysis that was presented at the San
Antonio Breast Cancer Symposium on the mortality data from the aromatase
inhibitor trials?
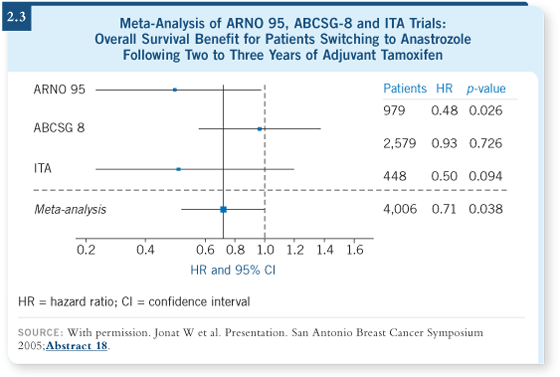
 DR PRITCHARD: The Austrian group presented an analysis of three of the
switching trials — the ITA trial, the ARNO 95 trial and the ABCSG-8 trial
— in which patients switched at two to three years from tamoxifen up front to
anastrozole ( Jonat 2005; [2.3]). The data showed a significant survival benefit
among those patients who were switched to anastrozole versus those who
remained on tamoxifen.
DR PRITCHARD: The Austrian group presented an analysis of three of the
switching trials — the ITA trial, the ARNO 95 trial and the ABCSG-8 trial
— in which patients switched at two to three years from tamoxifen up front to
anastrozole ( Jonat 2005; [2.3]). The data showed a significant survival benefit
among those patients who were switched to anastrozole versus those who
remained on tamoxifen.
 Track 13
Track 13
 DR LOVE: What are your thoughts about some of the recent data that
have come out in terms of cardiac issues and the aromatase inhibitors? DR LOVE: What are your thoughts about some of the recent data that
have come out in terms of cardiac issues and the aromatase inhibitors? |
 DR PRITCHARD: My view on this is probably controversial, but I think I’m
right. I believe we’re forgetting that tamoxifen is probably cardioprotective.
I’ve been in the game long enough to remember that when we started studying
tamoxifen as an adjuvant therapy, we knew it lowered lipids substantially, and
we all believed that it was going to show a cardiac effect that was beneficial.
DR PRITCHARD: My view on this is probably controversial, but I think I’m
right. I believe we’re forgetting that tamoxifen is probably cardioprotective.
I’ve been in the game long enough to remember that when we started studying
tamoxifen as an adjuvant therapy, we knew it lowered lipids substantially, and
we all believed that it was going to show a cardiac effect that was beneficial.
Indeed, the NSABP prevention study, P-1, was originally designed to examine
cardiac endpoints, but since the women who enrolled in the study were younger than expected, the cardiac component was dropped because the
cardiac rate was not expected to be high enough to answer the question.
The cardioprotective effect of tamoxifen was a big hypothesis, and when
you examine the available data, although they are a bit mixed, I think they
consistently show either no detriment or that there is a benefit with tamoxifen
compared to placebo or control.
Data from a study comparing five versus two years of tamoxifen were
presented at ASCO a couple of years ago by one of the Scandinavian groups
and were recently published in the Journal of Clinical Oncology. They showed
that the patients randomly assigned to five years of tamoxifen had a lower rate
of cardiac events and cardiac deaths. I believe that what we’re seeing in all of
these trials comparing aromatase inhibitors with tamoxifen is a small amount
of cardioprotection from tamoxifen.
In the MA17 trial, the only study in which the aromatase inhibitor is
compared to placebo, a lipid substudy shows virtually no difference in lipids or
cardiac events between the two groups. I think that there’s a protective effect
from the tamoxifen and maybe an extremely small effect, if any, from the
aromatase inhibitor, which I don’t think is substantial.
Select publications

