
| Tracks 1-20 |
| Track 1 |
Introduction |
| Track 2 |
Results from NSABP-B-31 and
NCCTG-N9831 trials of adjuvant
trastuzumab |
| Track 3 |
BCIRG 006: Adjuvant trastuzumab
with a nonanthracycline-containing
regimen |
| Track 4 |
Influence of TOPO II amplification
on the efficacy of anthracycline-based
chemotherapy in
BCIRG 006 |
| Track 5 |
Clinical implications of adjuvant
trastuzumab trials |
| Track 6 |
Use of Oncotype DX™ for
patients with HER2-positive
disease |
| Track 7 |
Delayed adjuvant trastuzumab |
| Track 8 |
Future directions for clinical
research in HER2-positive
disease |
| Track 9 |
Pilot trial of bevacizumab for
patients with inflammatory
breast cancer |
| Track 10 |
Predictors of response to
bevacizumab |
|
| Track 11 |
ECOG-E2100: Paclitaxel with
or without bevacizumab as
first-line therapy |
| Track 12 |
NSABP-B-38: Phase III trial
comparing three different
adjuvant chemotherapy regimens |
| Track 13 |
Benefit of dose-dense versus
nondose-dense chemotherapy in
the adjuvant setting |
| Track 14 |
Tolerability and efficacy of
ixabepilone for metastatic
breast cancer |
| Track 15 |
Clinical use of nanoparticle
albumin-bound (nab) paclitaxel |
| Track 16 |
Dosing of capecitabine |
| Track 17 |
Potential benefit of combining
pertuzumab and trastuzumab |
| Track 18 |
Management of inflammatory
breast cancer |
| Track 19 |
Tolerability of neoadjuvant
trastuzumab |
| Track 20 |
Future directions in the
management of breast cancer |
|
|
Select Excerpts from the Interview
 Track 2
Track 2
 DR LOVE: Could you review the initial adjuvant trastuzumab trials that
preceded BCIRG 006? DR LOVE: Could you review the initial adjuvant trastuzumab trials that
preceded BCIRG 006? |
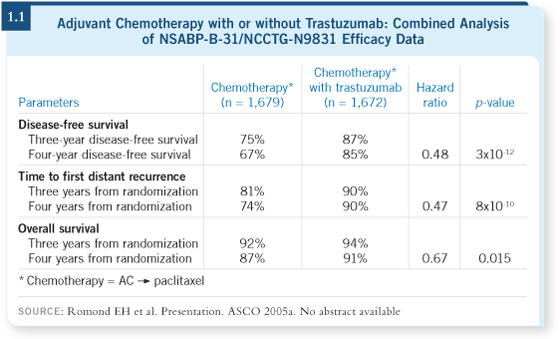
 DR SWAIN: At ASCO 2005, we heard presentations about three adjuvant
trastuzumab trials. One of the presentations was actually a combined analysis of NSABP-B-31 and NCCTG-N9831. I think everyone was stunned by the
data and the p-value of 10-12 showing efficacy when you add trastuzumab to
AC followed by paclitaxel (Romond 2005a; [1.1]). I was there, and I don’t
think there was a dry eye in the place. We have spent our lives working in
breast cancer research and have never seen a result like this. It was just
spectacular.
DR SWAIN: At ASCO 2005, we heard presentations about three adjuvant
trastuzumab trials. One of the presentations was actually a combined analysis of NSABP-B-31 and NCCTG-N9831. I think everyone was stunned by the
data and the p-value of 10-12 showing efficacy when you add trastuzumab to
AC followed by paclitaxel (Romond 2005a; [1.1]). I was there, and I don’t
think there was a dry eye in the place. We have spent our lives working in
breast cancer research and have never seen a result like this. It was just
spectacular.
After that, the HERA trial was presented, which had a little different design
in that it involved the sequential use of trastuzumab. That trial was also very
positive (Piccart-Gebhart 2005a). These were also data that were early, without
many events. It’s clear that this is changing the paradigm in breast cancer.
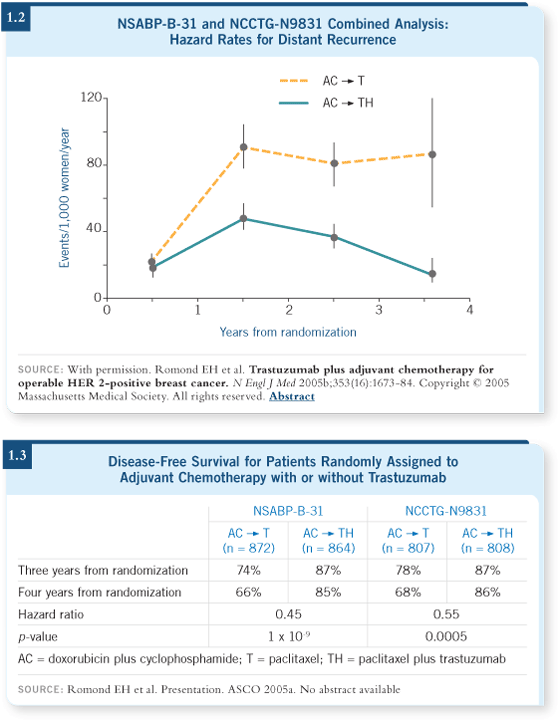
The hazard rates for distant recurrence in the combined analysis of NSABP-B-31 and NCCTG-N9831 looked as if they’re going down to almost zero. So we
may really be curing patients with the use of adjuvant trastuzumab (Romond
2005b; [1.2]).
 DR LOVE: In terms of combining the data from the two trials, some oncologists
were initially questioning whether that was legitimate. What are your
thoughts?
DR LOVE: In terms of combining the data from the two trials, some oncologists
were initially questioning whether that was legitimate. What are your
thoughts?
 DR SWAIN: The original study design was that each trial would look at the
disease-free survival separately. The groups got together before accrual was
completed for both of the studies and presented an analysis plan to the FDA in
order to get the results sooner. No one had any idea that we’d have the benefit
that we do, so they thought they needed the combined analysis to get some
results out there.
DR SWAIN: The original study design was that each trial would look at the
disease-free survival separately. The groups got together before accrual was
completed for both of the studies and presented an analysis plan to the FDA in
order to get the results sooner. No one had any idea that we’d have the benefit
that we do, so they thought they needed the combined analysis to get some
results out there.
When they did the combined analysis, it was so spectacular that the results
are still significant when the trials are analyzed separately (Romond 2005a;
[1.3]). They did not need to do a combined analysis, but they didn’t know that
beforehand. So I believe it was clearly legitimate.
 Track 3
Track 3
 DR LOVE: In September 2005, there was a press release about BCIRG
006. Then the data were presented at the 2005 San Antonio Breast Cancer
Symposium. Would you discuss that study? DR LOVE: In September 2005, there was a press release about BCIRG
006. Then the data were presented at the 2005 San Antonio Breast Cancer
Symposium. Would you discuss that study? |
 DR SWAIN: Dennis Slamon performed this courageous trial, in which he
chose to evaluate a nonanthracycline-containing regimen. He conducted the
pivotal metastatic trial of trastuzumab, so he was extremely concerned that AC followed by docetaxel/trastuzumab would cause cardiac toxicity. Therefore, he
wanted a treatment that did not involve an anthracycline.
DR SWAIN: Dennis Slamon performed this courageous trial, in which he
chose to evaluate a nonanthracycline-containing regimen. He conducted the
pivotal metastatic trial of trastuzumab, so he was extremely concerned that AC followed by docetaxel/trastuzumab would cause cardiac toxicity. Therefore, he
wanted a treatment that did not involve an anthracycline.
BCIRG 006 evaluated AC followed by docetaxel (T) with or without trastuzumab
(H) and TCH (docetaxel/carboplatin and trastuzumab). It was a scientifically
sound trial.
The interim analysis, conducted in September 2005, was presented at the 2005
San Antonio Breast Cancer Symposium. BCIRG 006 showed a strong benefit
associated with the use of trastuzumab — a 51 percent reduction in the risk of
recurrence for AC followed by docetaxel/trastuzumab and a 39 percent reduction
for TCH (Slamon 2005; [1.4]).
If you evaluated the three curves, AC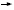 TH was on top, TCH was in the
middle and AC
TH was on top, TCH was in the
middle and AC T was on the bottom. There was not a statistically significant
difference if you compared AC
T was on the bottom. There was not a statistically significant
difference if you compared AC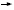 TH to TCH. However, if you look on
the graph, I think it’s clear that TCH is in the middle (Slamon 2005; [1.4]).
TH to TCH. However, if you look on
the graph, I think it’s clear that TCH is in the middle (Slamon 2005; [1.4]).
 Track 4
Track 4
 DR LOVE: Another fascinating data set Dr Slamon presented evaluated
topoisomerase II-alpha (TOPO II) amplification. Can you discuss that? DR LOVE: Another fascinating data set Dr Slamon presented evaluated
topoisomerase II-alpha (TOPO II) amplification. Can you discuss that? |
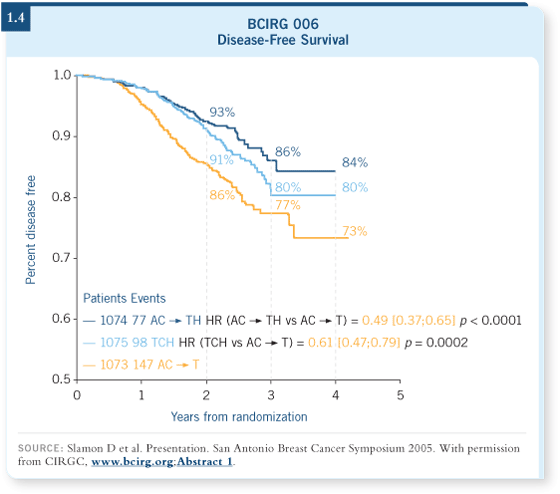
 DR SWAIN: TOPO II is on the same amplicon as HER2. Mike Press evaluated
TOPO II using FISH testing, and 35 percent of the tumors were
coamplified for TOPO II and HER2. They found that regardless of the treatment,
the patients who had TOPO II coamplification had a better prognosis
than those who did not (Slamon 2005).
DR SWAIN: TOPO II is on the same amplicon as HER2. Mike Press evaluated
TOPO II using FISH testing, and 35 percent of the tumors were
coamplified for TOPO II and HER2. They found that regardless of the treatment,
the patients who had TOPO II coamplification had a better prognosis
than those who did not (Slamon 2005).
Furthermore, if you evaluate the patients who did not have coamplified
tumors and look at the three treatment arms, you see that it didn’t seem
to matter whether they received TCH or AC TH. They all did better
compared to the nontrastuzumab-containing arm.
TH. They all did better
compared to the nontrastuzumab-containing arm.
In the smaller group, with the 35 percent of patients whose tumors were
coamplified, there was no significant difference between TCH and AC
followed by docetaxel (Slamon 2005; [1.5]). They’ve only looked at
approximately 2,000 out of the 3,000 patients, so these are preliminary
but exciting data.
 DR LOVE: The idea would be that if TOPO II is not amplified, you’re not
going to need an anthracycline and you won’t need to be exposed to the
cardiac risk?
DR LOVE: The idea would be that if TOPO II is not amplified, you’re not
going to need an anthracycline and you won’t need to be exposed to the
cardiac risk?
 DR SWAIN: That’s the interpretation a lot of people are discussing right now.
It is actually a great finding. If this were true, then TCH would be the treatment
of choice in those patients, because you obviously don’t want to expose
people to a cardiotoxic agent.
DR SWAIN: That’s the interpretation a lot of people are discussing right now.
It is actually a great finding. If this were true, then TCH would be the treatment
of choice in those patients, because you obviously don’t want to expose
people to a cardiotoxic agent.
They are extremely provocative data, and I’ve heard many people talking
about the need to check TOPO II in patients. But I believe right now, we
need to hold on. We have very early data with a very small number of events
when we start subgrouping. We need the rest of the data with more events,
and we need to look at them more carefully.
 Track 5
Track 5
 DR LOVE: Where do you think we are right now in terms of the clinical
application of these adjuvant trastuzumab data? DR LOVE: Where do you think we are right now in terms of the clinical
application of these adjuvant trastuzumab data? |
 DR SWAIN: From the four large trials that have been presented (Romond
2005a; Piccart-Gebhart 2005a; Slamon 2005) we have a lot of data
supporting the use of taxanes with trastuzumab. In clinical practice, the
best plan is to utilize the regimen that you are most comfortable with. AC
followed by weekly paclitaxel or by docetaxel are both effective treatments.
I personally would recommend every three-week docetaxel rather than
weekly docetaxel.
DR SWAIN: From the four large trials that have been presented (Romond
2005a; Piccart-Gebhart 2005a; Slamon 2005) we have a lot of data
supporting the use of taxanes with trastuzumab. In clinical practice, the
best plan is to utilize the regimen that you are most comfortable with. AC
followed by weekly paclitaxel or by docetaxel are both effective treatments.
I personally would recommend every three-week docetaxel rather than
weekly docetaxel.
However, I would not recommend vinorelbine. The FinHer study showed
that docetaxel had a better outcome compared to vinorelbine ( Joensuu 2005,
2006). I thought that was an interesting finding. With all of the other strong
data with the taxanes, a taxane would be my choice. I would use trastuzumab
concurrently with a taxane when possible.
 Track 5
Track 5
 DR LOVE: Would you summarize the data on patients with node-negative
disease from the adjuvant trastuzumab trials? DR LOVE: Would you summarize the data on patients with node-negative
disease from the adjuvant trastuzumab trials? |
 DR SWAIN: In NCCTG-N9831, about 10 percent of the patients had node-negative
disease (Romond 2005b), and none of those patients had tumors that
were less than a centimeter. Probably one of the questions I am asked the most
right now is, “What do you do with a patient who has a three-millimeter,
HER2-positive tumor?”
DR SWAIN: In NCCTG-N9831, about 10 percent of the patients had node-negative
disease (Romond 2005b), and none of those patients had tumors that
were less than a centimeter. Probably one of the questions I am asked the most
right now is, “What do you do with a patient who has a three-millimeter,
HER2-positive tumor?”
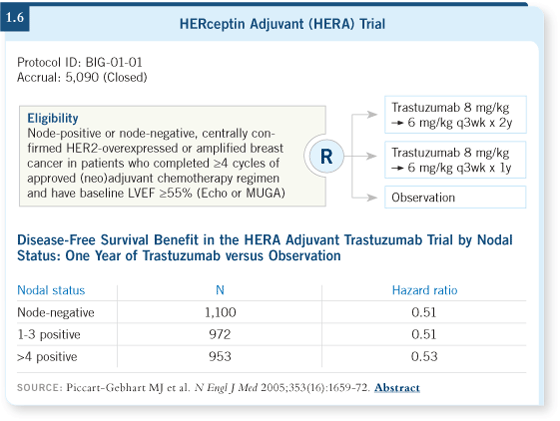
In BCIRG 006, about 30 percent of the patients had node-negative disease
(Slamon 2005), and some of those patients did have very small tumors. There
was not a size limitation, but it’s still, again, limited in number. In the HERA
trial, approximately 30 percent of the patients had node-negative disease. The
HERA trial was very strongly positive for efficacy with sequential trastuzumab
in the patients with node-negative disease (Piccart-Gebhart 2005a; [1.6]).
Hence, strong data support the use of adjuvant trastuzumab in patients with
node-negative disease. The nuances of its use are really about the tiny tumors.
Do we need to treat those? I attended a meeting last night with Dennis
Slamon, and he believes that even patients with the smallest HER2-positive
tumors should receive trastuzumab.
I disagree with that a little bit because the data indicate that a patient with a
two- or three-millimeter tumor has an extremely good prognosis. I have not
been recommending adjuvant trastuzumab for those very tiny tumors because
of the risk of cardiac toxicity. It’s not like tamoxifen, with which you have
minimal risk. You do have risk, and it requires intravenous therapy for a year.
 Track 6
Track 6
 DR LOVE: What about the use of trastuzumab monotherapy in the patient
whom you wouldn’t want to treat with chemotherapy because of age and
comorbid illnesses, perhaps a patient with an ER-negative tumor? DR LOVE: What about the use of trastuzumab monotherapy in the patient
whom you wouldn’t want to treat with chemotherapy because of age and
comorbid illnesses, perhaps a patient with an ER-negative tumor? |
 DR SWAIN: I probably wouldn’t use trastuzumab without chemotherapy. Most
likely, even if there’s comorbidity, paclitaxel can be tolerated very well if you
use it weekly.
DR SWAIN: I probably wouldn’t use trastuzumab without chemotherapy. Most
likely, even if there’s comorbidity, paclitaxel can be tolerated very well if you
use it weekly.
Such a large amount of synergy data exists with trastuzumab, even though the
HERA trial is positive with sequential use (Piccart-Gebhart 2005b), I believe
Dennis Slamon’s laboratory data that indicate the synergy is important.
So I would try to use paclitaxel with trastuzumab in those patients, and if
you’re concerned about the anthracycline, just don’t use that.
 Track 7
Track 7
 DR LOVE: Another common question that comes up is about the patient
with HER2-positive, node-positive disease who’s been treated with
adjuvant chemotherapy in the past — six months or a couple of years ago.
What are your thoughts about guidelines for those patients? DR LOVE: Another common question that comes up is about the patient
with HER2-positive, node-positive disease who’s been treated with
adjuvant chemotherapy in the past — six months or a couple of years ago.
What are your thoughts about guidelines for those patients? |
 DR SWAIN: Generally, if it’s six months or less, I follow the guidelines from
the trials and offer therapy. I have occasionally used trastuzumab in one or
two patients who were farther out than that if they had high-risk disease. I
think it’s reasonable with such a huge benefit.
DR SWAIN: Generally, if it’s six months or less, I follow the guidelines from
the trials and offer therapy. I have occasionally used trastuzumab in one or
two patients who were farther out than that if they had high-risk disease. I
think it’s reasonable with such a huge benefit.
 DR LOVE: Do you think it makes sense to try to estimate what the chance of
recurrence is from that point in time for a patient who is out a year or two or
more from her initial treatment?
DR LOVE: Do you think it makes sense to try to estimate what the chance of
recurrence is from that point in time for a patient who is out a year or two or
more from her initial treatment?
 DR SWAIN: I think the way I would look at it is exactly what you said. You
take the risk that they had initially, a year ago, and the benefit of whatever
you gave them to see where they are at that point. I think it is reasonable to
do that, and you have to do that. I don’t know how else you could do it.
DR SWAIN: I think the way I would look at it is exactly what you said. You
take the risk that they had initially, a year ago, and the benefit of whatever
you gave them to see where they are at that point. I think it is reasonable to
do that, and you have to do that. I don’t know how else you could do it.
Select publications

