
 |
||||||||

| Tracks 1-16 | ||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
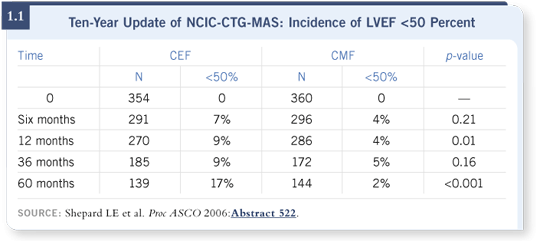
![]() DR GRALOW: Lois Shepherd presented long-term follow-up data from the
NCIC-CTG-MA5 trial comparing CEF versus CMF (Shepherd 2006).
Clearly cardiomyopathy is real, and it occurs more often in patients who
receive anthracycline-containing regimens (1.1).
DR GRALOW: Lois Shepherd presented long-term follow-up data from the
NCIC-CTG-MA5 trial comparing CEF versus CMF (Shepherd 2006).
Clearly cardiomyopathy is real, and it occurs more often in patients who
receive anthracycline-containing regimens (1.1).
The trials remind us that we need to consider toxicities when we’re offering patients only a couple of percentage points' improvement in survival. For patients at low risk, that small improvement has to be weighed against serious toxicities such as congestive heart failure and acute leukemia, even though they are rare.
![]() DR LOVE: How would you counsel a patient in the adjuvant setting regarding
her risk of developing a doxorubicin-related cardiomyopathy, and has that
changed since the ASCO meeting?
DR LOVE: How would you counsel a patient in the adjuvant setting regarding
her risk of developing a doxorubicin-related cardiomyopathy, and has that
changed since the ASCO meeting?
![]() DR GRALOW: For patients receiving four doses of doxorubicin at 60 mg/m2,
in the past I would have quoted a one percent or less chance of developing
symptomatic cardiomyopathy. After ASCO, I would tell patients that with
long-term follow-up, the risk could be as high as a couple of percentage
points, even with the 240 mg/m2 dose and especially when increasing doxorubicin
to 360 mg/m2, as they have done in some of the studies.
DR GRALOW: For patients receiving four doses of doxorubicin at 60 mg/m2,
in the past I would have quoted a one percent or less chance of developing
symptomatic cardiomyopathy. After ASCO, I would tell patients that with
long-term follow-up, the risk could be as high as a couple of percentage
points, even with the 240 mg/m2 dose and especially when increasing doxorubicin
to 360 mg/m2, as they have done in some of the studies.
Clearly, hypertension and other risk factors for cardiac disease in general are risk factors for chemotherapy-related heart toxicity, and although everything was done correctly with Dr Dresdner’s patient (1.2) in that the ejection fraction was checked before treatment, standard doses were used and the cumulative dose was quite low, this case illustrates that occasionally cardiomyopathy can happen even in a patient who does not appear to be at high risk.

![]() DR LOVE: What do we know about the clinical course of anthracycline-related
cardiomyopathy, particularly in the adjuvant setting?
DR LOVE: What do we know about the clinical course of anthracycline-related
cardiomyopathy, particularly in the adjuvant setting?
![]() DR GRALOW: Asymptomatic drops in ejection fractions certainly do occur
while patients are on therapy, but symptomatic congestive heart failure doesn’t
usually occur until after the chemotherapy is completed. It can be a couple
of years later or even out to seven years. We do know from the trastuzumab
trials that many drops in ejection fraction with AC prevented patients from
receiving a taxane and trastuzumab.
DR GRALOW: Asymptomatic drops in ejection fractions certainly do occur
while patients are on therapy, but symptomatic congestive heart failure doesn’t
usually occur until after the chemotherapy is completed. It can be a couple
of years later or even out to seven years. We do know from the trastuzumab
trials that many drops in ejection fraction with AC prevented patients from
receiving a taxane and trastuzumab.
![]() DR LOVE: In your practice, for which patients with HER2-negative disease
receiving an anthracycline do you obtain pretreatment ejection fractions?
DR LOVE: In your practice, for which patients with HER2-negative disease
receiving an anthracycline do you obtain pretreatment ejection fractions?
![]() DR GRALOW: In general, I always obtain an ejection fraction on patients over
the age of 60. I don’t routinely do so for younger patients with no known risk
factors, although that is variable because I do discuss with patients the risk of
cardiac toxicities, and some want to know their baseline measurement.
I don’t generally repeat the ejection fraction for asymptomatic patients unless
they are on trastuzumab.
DR GRALOW: In general, I always obtain an ejection fraction on patients over
the age of 60. I don’t routinely do so for younger patients with no known risk
factors, although that is variable because I do discuss with patients the risk of
cardiac toxicities, and some want to know their baseline measurement.
I don’t generally repeat the ejection fraction for asymptomatic patients unless
they are on trastuzumab.
Track 2
![]() DR GRALOW: I would prefer not to have to give an anthracycline. The risk
of myelodysplasia, acute leukemia and congestive heart failure with anthracyclines
are all real concerns, especially for patients with node-negative disease
who are receiving only a small benefit from chemotherapy.
DR GRALOW: I would prefer not to have to give an anthracycline. The risk
of myelodysplasia, acute leukemia and congestive heart failure with anthracyclines
are all real concerns, especially for patients with node-negative disease
who are receiving only a small benefit from chemotherapy.
I’m not a huge a fan of CMF, although some of my colleagues still feel there is a time and place for this regimen. We have some nice ongoing studies investigating replacing anthracyclines, and many trials suggest that some better regimens exist for this group of patients as a whole.
A subpopulation that does just fine with CMF probably exists, but I also wonder if that’s not a group that would also do just as well with endocrine therapy alone, especially with better endocrine therapy.
![]() DR LOVE: What adjuvant studies are currently evaluating nonanthracycline
regimens?
DR LOVE: What adjuvant studies are currently evaluating nonanthracycline
regimens?
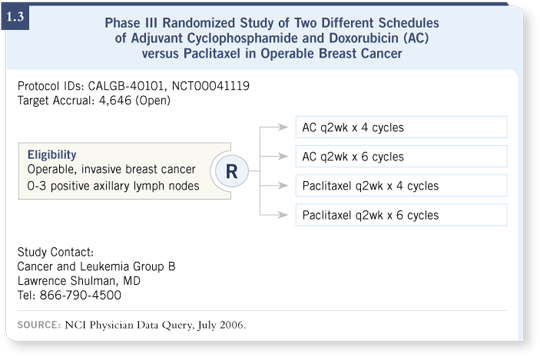
![]() DR GRALOW: CALGB has a four-arm trial, 40101, examining AC versus
paclitaxel for patients with only one to three positive nodes or node-negative
disease (1.3).
DR GRALOW: CALGB has a four-arm trial, 40101, examining AC versus
paclitaxel for patients with only one to three positive nodes or node-negative
disease (1.3).
The AC and paclitaxel are given every two weeks with growth factors for either four cycles or six cycles. That’s an important study, evaluating whether we can give these patients only a taxane.
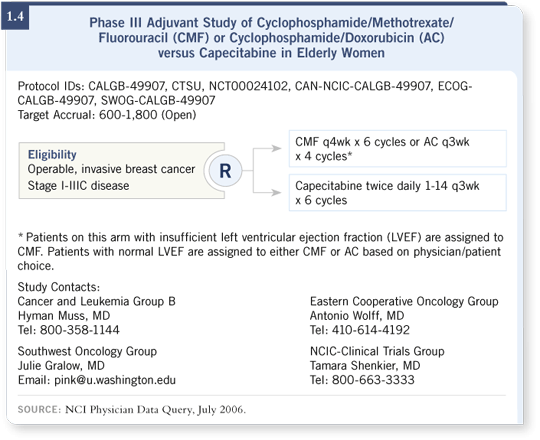
In the older population, Hy Muss is conducting the CALGB-49907 trial (1.4), which randomly assigns women older than age 65 to single-agent capecitabine versus AC or CMF, the physician’s choice.
I believe these are both great studies that may prove that a single agent without a lot of CHF and leukemia risk is an appropriate substitute for standard chemotherapy regimens, especially for women in the lower-risk group.
![]() DR LOVE: What do you think CALGB-49907 will show with regard to
toxicities and efficacy?
DR LOVE: What do you think CALGB-49907 will show with regard to
toxicities and efficacy?
![]() DR GRALOW: Initially the capecitabine was given at a full dose, and two early
deaths occurred among the patients on capecitabine. One clearly looked like a
DPD deficiency, and the other probably was the same, although it took a little
longer for the symptoms to develop.
DR GRALOW: Initially the capecitabine was given at a full dose, and two early
deaths occurred among the patients on capecitabine. One clearly looked like a
DPD deficiency, and the other probably was the same, although it took a little
longer for the symptoms to develop.
At that point, some safeguards were added and we were allowed to reduce the dose. We have not had a problem with deaths or serious issues subsequently.
The trial is designed to evaluate whether capecitabine is superior, and it has a real chance of showing that. Capecitabine as a single agent in the metastatic setting is a great drug, so it could win.
In terms of the long-term toxicities, I expect the patients who receive AC will have some cardiac toxicity and potentially leukemia at some point in their lifetime. Even though this study involves an older population, we’re living so long now that women who are 65 could live another three decades.
Track 4
![]() DR GRALOW: I’m much more likely to use a gentler chemotherapy regimen
for older patients, and I use capecitabine for patients who have some comorbidities
or a little lower performance status. Otherwise, I’m not sure I’m ready
to make that leap for my younger patients without further data comparing it to
an anthracycline/taxane combination, for example.
DR GRALOW: I’m much more likely to use a gentler chemotherapy regimen
for older patients, and I use capecitabine for patients who have some comorbidities
or a little lower performance status. Otherwise, I’m not sure I’m ready
to make that leap for my younger patients without further data comparing it to
an anthracycline/taxane combination, for example.
A group of women might exist who would prefer to avoid the side effects of standard chemotherapy, specifically someone who wants to keep working and doesn’t want to lose her hair because she doesn’t want people to know she’s on treatment and doesn’t want the lengthy appointments in the infusion room. It’s an interesting option, and I’m optimistic that it will be a choice for some of our patients in the near future.
Track 6
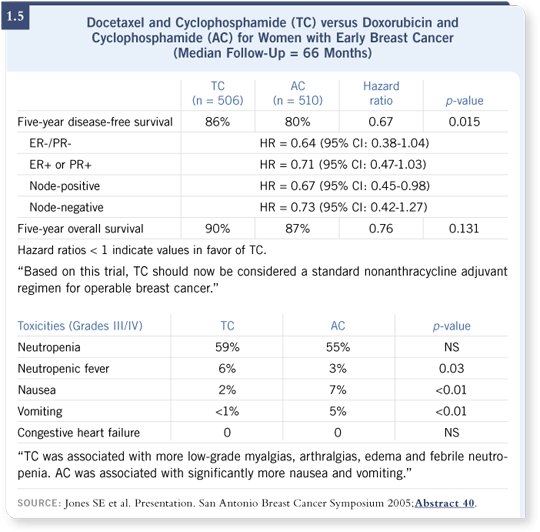
![]() DR GRALOW: That presentation was striking (1.5). The trial’s toxicity data
showed that TC wasn’t substantially more toxic than AC (1.5). I’ve never
prescribed TC, but if I were considering AC for a patient, TC would be a
reasonable alternative. No survival advantage appeared that was significant,
but a disease-free survival advantage was evident, and it was clinically relevant.
DR GRALOW: That presentation was striking (1.5). The trial’s toxicity data
showed that TC wasn’t substantially more toxic than AC (1.5). I’ve never
prescribed TC, but if I were considering AC for a patient, TC would be a
reasonable alternative. No survival advantage appeared that was significant,
but a disease-free survival advantage was evident, and it was clinically relevant.
Track 7
![]() DR GRALOW: The five-year bone density substudy of the ATAC trial was very
interesting. The fracture rates on that trial were approximately 11 percent in
the anastrozole arm and about 7.5 percent in the tamoxifen arm at 68 months
of follow-up (Howell 2005).
DR GRALOW: The five-year bone density substudy of the ATAC trial was very
interesting. The fracture rates on that trial were approximately 11 percent in
the anastrozole arm and about 7.5 percent in the tamoxifen arm at 68 months
of follow-up (Howell 2005).
However, we were trying to determine who should receive bisphosphonates up front and how often we should follow bone density studies. I believe the ATAC data that Rob Coleman presented at ASCO showed that not everyone needs a DEXA scan every year or a bisphosphonate up front (Coleman 2006; [1.6]).
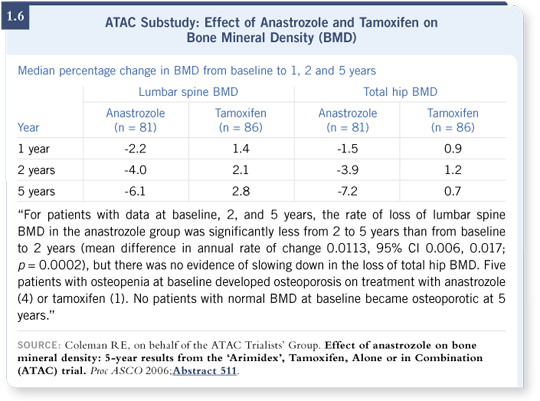
What was surprising to me but very reassuring was that none of the patients who started the ATAC trial with a normal bone mineral density — a T-score better than minus one — were osteoporotic after five years of treatment, although approximately 50 percent had become osteopenic.
We expect about a two to three percent bone loss during the five years simply based on aging, but in the tamoxifen arm, approximately 15 to 20 percent of the patients went from normal to osteopenic, and the rate was 50 percent for patients who received anastrozole.
Aging happens even to the best of us, but I believe these data show us that if the patient started with a normal bone mineral density, her chance of becoming osteoporotic after five years as a result of receiving an aromatase inhibitor in that study was zero.
Track 14
![]() DR GRALOW: The aromatase inhibitors continue to be an evolving story, with
the first survival differences now being reported. The IES and ARNO 95 trials show a benefit to the patients who switched to an aromatase inhibitor
(Coombes 2006; Kaufman 2006).
DR GRALOW: The aromatase inhibitors continue to be an evolving story, with
the first survival differences now being reported. The IES and ARNO 95 trials show a benefit to the patients who switched to an aromatase inhibitor
(Coombes 2006; Kaufman 2006).
The question becomes whether priming occurs with tamoxifen or whether efficacy is improved because the two drugs are not totally cross-reactive in terms of resistance.
![]() DR LOVE: Is it possible a more favorable group of patients with more
endocrine-responsive tumors makes it to two years?
DR LOVE: Is it possible a more favorable group of patients with more
endocrine-responsive tumors makes it to two years?
![]() DR GRALOW: Yes. All of these are potential reasons for why we’re seeing
a survival advantage first in the switching trials rather than with up-front
aromatase inhibitors.
DR GRALOW: Yes. All of these are potential reasons for why we’re seeing
a survival advantage first in the switching trials rather than with up-front
aromatase inhibitors.
We saw an update of the MA17 trial examining the patients who originally received a placebo after five years of tamoxifen as opposed to letrozole and then at about 30 months, when the study was unblinded, were offered letrozole (Robert 2006). Approximately two thirds of those patients chose letrozole, and they tended to be a higher-risk group.
Those patients had an average gap of 30 months without any endocrine therapy. Despite that and the fact that they were a good eight years out from their diagnosis, a reduction appeared across the board in every type of breast cancer recurrence — contralateral, in-breast and distant. It’s impressive.
We saw the updated analysis for the MA17 trial at the San Antonio meeting in 2005 (Goss 2005), and at that point I began to at least offer patients the option of going back on an endocrine agent if they’d been off everything for a couple of years, especially if they were at high risk.
![]() DR LOVE: How long off tamoxifen or how long past her breast cancer
diagnosis are you now willing to treat someone? If you saw a patient who was
treated 10 years ago, would you discuss this option?
DR LOVE: How long off tamoxifen or how long past her breast cancer
diagnosis are you now willing to treat someone? If you saw a patient who was
treated 10 years ago, would you discuss this option?
![]() DR GRALOW: Probably not. Although it might offer some benefit 10 years
later, the duration off therapy in the MA17 trial was approximately 30 months,
so I consider restarting endocrine therapy for patients up to three years off
treatment. That’s arbitrary, but you have to pick some time period.
DR GRALOW: Probably not. Although it might offer some benefit 10 years
later, the duration off therapy in the MA17 trial was approximately 30 months,
so I consider restarting endocrine therapy for patients up to three years off
treatment. That’s arbitrary, but you have to pick some time period.