
 |
||||||||
Miami Breast Cancer Conference Tumor Panel:
Monoclonal Antibodies
in the Management of Early and Advanced Breast Cancer
| Tracks 1-19 | ||||||||||||||||||||||||||||||||||||||||
|
Monoclonal Antibody Therapy in the Metastatic Setting

Track 6
![]() DR SLEDGE: The first issue that one considers for a patient with metastatic
breast cancer is prior therapy. This patient has received prior anthracycline-based
chemotherapy but not taxane-based chemotherapy. So we would
certainly say that taxane-based therapy would be a standard of care for this
patient.
DR SLEDGE: The first issue that one considers for a patient with metastatic
breast cancer is prior therapy. This patient has received prior anthracycline-based
chemotherapy but not taxane-based chemotherapy. So we would
certainly say that taxane-based therapy would be a standard of care for this
patient.
I’ll add another qualifier, which is that I’m not always sure we obtain correct “steroid” or HER2 receptor values. When you have a patient who has relapsed, you can almost always make a case for obtaining more tissue and retesting for ER, PR and HER2 because a small but fixed percentage of the time you’ll find out that your pathologist didn’t quite do it right in the past.
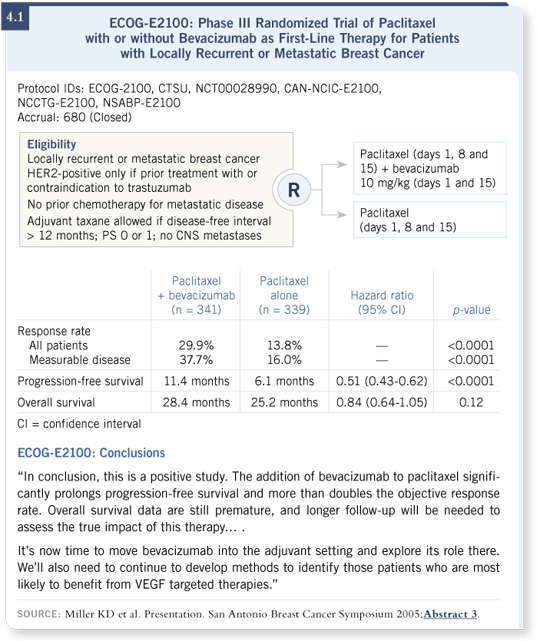
Let’s assume this patient has triple-negative disease. In my clinic, based on the results of ECOG-E2100, we would likely recommend that she receive combination therapy with weekly paclitaxel and every two-week bevacizumab. ECOG-E2100 randomly assigned similar patients to receive chemotherapy alone — paclitaxel — or chemotherapy and bevacizumab (Miller 2005; [4.1]).
The results from ECOG-E2100 demonstrated that bevacizumab increased the median progression-free survival from about six months to about 11 months (Miller 2005; [4.1]). This five-month improvement is the largest improvement we’ve seen in a generation for progression-free survival among patients with metastatic breast cancer. It is, for instance, a larger absolute improvement than the one seen with trastuzumab in the trial comparing paclitaxel with or without trastuzumab as front-line therapy (Slamon 2001).
Track 7
![]() DR WINER: The study was essentially open to women with HER2-negative
disease, although it did include a handful of patients with HER2-positive
disease who had received trastuzumab on a preoperative or adjuvant pilot
study. Two thirds of the women in the study had ER-positive breast cancer
and one third had ER-negative breast cancer (Miller 2005). Because most had
HER2-negative disease, many had triple-negative disease like the patient in
this case.
DR WINER: The study was essentially open to women with HER2-negative
disease, although it did include a handful of patients with HER2-positive
disease who had received trastuzumab on a preoperative or adjuvant pilot
study. Two thirds of the women in the study had ER-positive breast cancer
and one third had ER-negative breast cancer (Miller 2005). Because most had
HER2-negative disease, many had triple-negative disease like the patient in
this case.
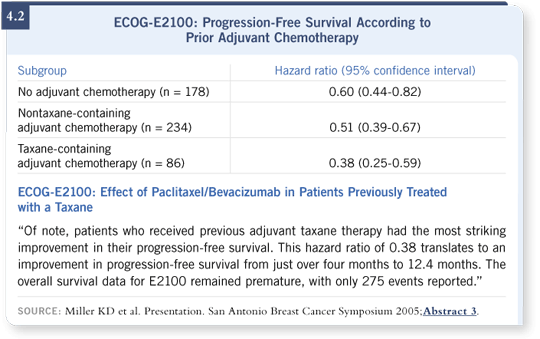
Apart from that, the study was relatively permissive. Most, but not all, of the patients had not received a taxane, like this woman. Of interest, although the group was small, the women who had received a taxane in the adjuvant setting appeared to derive every bit as much benefit from bevacizumab as those who hadn’t (Miller 2005; [4.2]). Of course, the investigators looked for CNS disease because of concerns about bleeding. Hence, all the women on ECOGE2100 had a negative scan of their brain.
I believe the real issue in terms of how one thinks about this patient is the sense that she’s going to have more difficulty soon. She has triple-negative disease, and she’s had a short disease-free interval. She’s not terribly symptomatic, but at the same time, I would expect she would be more symptomatic in the short term.
The treatment options for somebody in this situation are relatively limited. I personally agree with George. For this patient, I would say it’s a “slam-dunk” decision that she should receive bevacizumab and paclitaxel. Unless she’s eligible for some trial that you want to pursue, that is the standard treatment for this woman.
Track 8
![]() DR WINER: I’m not sure that I would because I view bevacizumab plus paclitaxel
as combination therapy. I don’t know that adding another chemotherapy
drug will do that much more. The paradox of the patient who’s in visceral
crisis and sicker is that although on the one hand one is sometimes tempted
to add another drug — and sometimes I do that — this is also the patient for
whom you’re even more concerned about adding toxicities from drugs because
she may become that much sicker as a result of the drugs.
DR WINER: I’m not sure that I would because I view bevacizumab plus paclitaxel
as combination therapy. I don’t know that adding another chemotherapy
drug will do that much more. The paradox of the patient who’s in visceral
crisis and sicker is that although on the one hand one is sometimes tempted
to add another drug — and sometimes I do that — this is also the patient for
whom you’re even more concerned about adding toxicities from drugs because
she may become that much sicker as a result of the drugs.
![]() DR LOVE: Joyce, what would likely be your therapy for Dr Lowenthal’s
patient?
DR LOVE: Joyce, what would likely be your therapy for Dr Lowenthal’s
patient?
![]() DR O’SHAUGHNESSY: Outside of a clinical trial, I would treat her with
weekly paclitaxel and bevacizumab. We happen to have a very interesting
clinical trial that I’ll mention. It is randomly assigning patients with triple-negative
disease to irinotecan/carboplatin with or without weekly cetuximab,
because about half of the patients with triple-negative disease have EGFR
expression. We don’t know if it’s driving the cancer at all. This is a randomized
Phase II trial to see if there’s any signal that response rates or time to
progression will be better in this group with cetuximab. So I would also talk
to her about that clinical trial.
DR O’SHAUGHNESSY: Outside of a clinical trial, I would treat her with
weekly paclitaxel and bevacizumab. We happen to have a very interesting
clinical trial that I’ll mention. It is randomly assigning patients with triple-negative
disease to irinotecan/carboplatin with or without weekly cetuximab,
because about half of the patients with triple-negative disease have EGFR
expression. We don’t know if it’s driving the cancer at all. This is a randomized
Phase II trial to see if there’s any signal that response rates or time to
progression will be better in this group with cetuximab. So I would also talk
to her about that clinical trial.
Track 9
![]() DR WINER: It might have been single-agent paclitaxel or capecitabine. It
would have almost certainly been some single-agent chemotherapy. I don’t
believe the order in which we use these drugs makes a big difference. The
reason I’d pick paclitaxel now is that it was used in ECOG-E2100, and I have
no reason not to use paclitaxel for this patient.
DR WINER: It might have been single-agent paclitaxel or capecitabine. It
would have almost certainly been some single-agent chemotherapy. I don’t
believe the order in which we use these drugs makes a big difference. The
reason I’d pick paclitaxel now is that it was used in ECOG-E2100, and I have
no reason not to use paclitaxel for this patient.
![]() DR SLEDGE: I would have used a single-agent taxane — either paclitaxel on a
weekly basis or docetaxel on an every three-week basis.
DR SLEDGE: I would have used a single-agent taxane — either paclitaxel on a
weekly basis or docetaxel on an every three-week basis.
![]() DR O’SHAUGHNESSY: I tend to use combination therapy early on, and I
probably would have used either paclitaxel or docetaxel in combination with
capecitabine. I like the strategy of obtaining the highest possible response rate
and trying to consolidate with some radiation therapy.
DR O’SHAUGHNESSY: I tend to use combination therapy early on, and I
probably would have used either paclitaxel or docetaxel in combination with
capecitabine. I like the strategy of obtaining the highest possible response rate
and trying to consolidate with some radiation therapy.
Stopping the taxane and continuing indefinitely with capecitabine is a strategy I use a lot. You administer about three to four months of the combination and then stop the taxane.
![]() DR LOVE: You’ve changed your paradigm pretty significantly.
DR LOVE: You’ve changed your paradigm pretty significantly.
![]() DR O’SHAUGHNESSY: Yes. The bevacizumab data, with the prolonged time
to progression, are very intriguing. This patient, unfortunately, is at significant
risk for brain metastases. It will be intriguing in ECOG-E2100 to see if
any difference emerges, ultimately, in the development of brain metastases by
inhibiting VEGF. I don’t know the answer to that, but it would be terrific if
that were the case.
DR O’SHAUGHNESSY: Yes. The bevacizumab data, with the prolonged time
to progression, are very intriguing. This patient, unfortunately, is at significant
risk for brain metastases. It will be intriguing in ECOG-E2100 to see if
any difference emerges, ultimately, in the development of brain metastases by
inhibiting VEGF. I don’t know the answer to that, but it would be terrific if
that were the case.
![]() DR LOVE: Dr Lowenthal, can you follow up with this case?
DR LOVE: Dr Lowenthal, can you follow up with this case?
![]() DR LOWENTHAL: In light of ECOG 2100, we would have liked to have
started her on paclitaxel and bevacizumab, but appeals to her insurance
company were repeatedly rejected.
DR LOWENTHAL: In light of ECOG 2100, we would have liked to have
started her on paclitaxel and bevacizumab, but appeals to her insurance
company were repeatedly rejected.
We ultimately started her on single-agent paclitaxel, weekly, and she did well at first. A diminution in the tumor markers occurred, and there was a diminution in her palpable disease for about the first six to seven weeks, after which things slowly started to pick up again.
I restaged her a few days ago because her markers were going up and her performance level was slipping. A PET scan and a CAT scan showed that the disease was taking quite a leap. Her mediastinal nodal disease is now very extensive, and her chest wall lesion has doubled in size.
She has extensive bone metastases, which she did not have at the start of treatment. Because of the tempo of the disease, we bit the bullet and I switched her to docetaxel/capecitabine and bevacizumab.

Track 10
![]() DR WINER: In the end, I would probably treat her the same as the prior
patient, although I think about her a little differently. She’s asymptomatic,
but she has bulky liver disease. That would make me hesitant about using
a hormonal agent, although she may have hormone-responsive disease. So
I would start with some form of chemotherapy. Based on ECOG-E2100, I
would probably start with bevacizumab and paclitaxel, since it worked
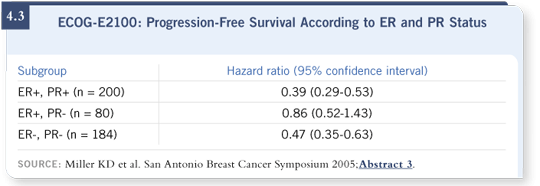
equally well for ER-positive disease (Miller 2005; [4.3]).
DR WINER: In the end, I would probably treat her the same as the prior
patient, although I think about her a little differently. She’s asymptomatic,
but she has bulky liver disease. That would make me hesitant about using
a hormonal agent, although she may have hormone-responsive disease. So
I would start with some form of chemotherapy. Based on ECOG-E2100, I
would probably start with bevacizumab and paclitaxel, since it worked
equally well for ER-positive disease (Miller 2005; [4.3]).
I’m more optimistic about what’s open to her in the future. If you told me she had two 2- or 3-cm lesions in her liver and normal liver function test results, I would have been quick to say, “Hold off the chemotherapy and use a hormonal agent.”
![]() DR LOVE: George, how would you approach this woman?
DR LOVE: George, how would you approach this woman?
![]() DR SLEDGE: Pretty much the same way as Eric would. I hear about visceral
crisis a lot, but I don’t see it much in my clinic. In fact, most of our patients
with metastatic breast cancer do not fall into the category of visceral metastases.
DR SLEDGE: Pretty much the same way as Eric would. I hear about visceral
crisis a lot, but I don’t see it much in my clinic. In fact, most of our patients
with metastatic breast cancer do not fall into the category of visceral metastases.
Medicine is never going to be boiled down to simple rules. All of us, as physicians, when we’re in a room with a patient, have a gestalt about the patient and our comfort level about whether the patient needs hormonal therapy or chemotherapy. This is a patient who I believe, as Eric has suggested, is right on the borderline.
![]() DR LOVE: Joyce, briefly, how would you treat this patient?
DR LOVE: Joyce, briefly, how would you treat this patient?
![]() DR O’SHAUGHNESSY: With this ER-positive biology that relapses in the
bone and/or the liver — the primary sites of metastases — I’m extraordinarily
impressed with the taxane/capecitabine combinations. We’ve done a lot of
studies with either docetaxel/capecitabine or weekly paclitaxel/capecitabine. I
have four patients with complete responses who are more than three years out,
and they all have the same biology, exactly like this patient.
DR O’SHAUGHNESSY: With this ER-positive biology that relapses in the
bone and/or the liver — the primary sites of metastases — I’m extraordinarily
impressed with the taxane/capecitabine combinations. We’ve done a lot of
studies with either docetaxel/capecitabine or weekly paclitaxel/capecitabine. I
have four patients with complete responses who are more than three years out,
and they all have the same biology, exactly like this patient.
So I probably would not use bevacizumab for this patient because of my own experience with the taxane/capecitabine combinations. I’d be interested in using bevacizumab with her next round of therapy. It’s an excellent agent, and it looks very promising, but with this particular biology, I’m extremely impressed with the taxane/capecitabine combinations.

Track 11
![]() DR HARWIN: We ended up treating her with paclitaxel and bevacizumab. She
had a very nice partial response. She received about nine or 10 cycles with
very little toxicity except that she started to develop some neuropathy. Because
of the neuropathy and the plateau in her response, I decided to stop the treatment
and switched her to letrozole.
DR HARWIN: We ended up treating her with paclitaxel and bevacizumab. She
had a very nice partial response. She received about nine or 10 cycles with
very little toxicity except that she started to develop some neuropathy. Because
of the neuropathy and the plateau in her response, I decided to stop the treatment
and switched her to letrozole.
Track 15
![]() DR SLEDGE: I believe it’s safe to say that immunohistochemistry (IHC) represents
an art form, which is to say that we ask the pathologist to look under
the microscope and determine if something is zero, 1+, 2+ or 3+. Variability
occurs among pathologists and with the same pathologist from day to day.
DR SLEDGE: I believe it’s safe to say that immunohistochemistry (IHC) represents
an art form, which is to say that we ask the pathologist to look under
the microscope and determine if something is zero, 1+, 2+ or 3+. Variability
occurs among pathologists and with the same pathologist from day to day.
It’s reasonably likely that about 15 or 20 percent of the time the average pathologist just gets it wrong. Problems also arise related to fixation, which antibody is used, et cetera. Even in “good hands,” a fair amount of interpathologist and intrapathologist variability occurs.
Fluorescence in situ hybridization (FISH), in theory, should overcome those problems. When you do a FISH test, you have an internal control in the form of the chromosome 17-centromere marker. You should be able to count the number of centromeres and the number of HER2 in the cell and obtain a ratio that should be absolutely perfect.
Of course, in the real world, nothing is perfect. Indeed, emerging data suggest — because FISH has to be done fairly quickly and pathologists are not all equally well trained — that false-positive and false-negative results occur with FISH (Perez 2006). This is difficult for all of us, but I expect it will get better over time.
Today in my hospital, we tend to use FISH pretty much exclusively. I would not criticize someone if they had a patient with a result of 2+ by immunohistochemistry and then did a FISH test on that patient’s tumor, or if they ignored FISH for a patient with a result of 3+ by IHC.
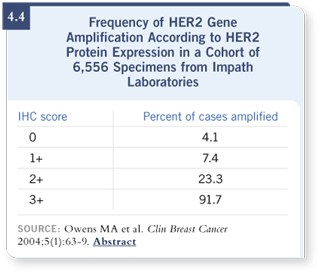 The tougher area includes those patients with a result of 0 and 1+, because
we know from a fair number of studies that some of those patients have
FISH-positive disease — about four and seven percent, respectively, in the
largest database (Owens
2004; [4.4]).
The tougher area includes those patients with a result of 0 and 1+, because
we know from a fair number of studies that some of those patients have
FISH-positive disease — about four and seven percent, respectively, in the
largest database (Owens
2004; [4.4]).
Do you want to ignore seven percent of the patients with an IHC of 1+ when their lives might be saved by HER2 testing with FISH? I don’t know the answer to that. Part of it is a cost-benefit analysis, and part of it is availability in your own hospital. In my hospital, however, we typically would use FISH.
Track 16
![]() DR O’SHAUGHNESSY: We don’t have good data sets to tell us how effective
hormonal therapy alone (ie, tamoxifen) would be for this premenopausal
woman. I don’t feel comfortable using our overview analysis, in which we can
show a 40 or 50 percent reduction in the risk of relapse with tamoxifen for all
comers (EBCTCG 2005).
DR O’SHAUGHNESSY: We don’t have good data sets to tell us how effective
hormonal therapy alone (ie, tamoxifen) would be for this premenopausal
woman. I don’t feel comfortable using our overview analysis, in which we can
show a 40 or 50 percent reduction in the risk of relapse with tamoxifen for all
comers (EBCTCG 2005).
I don’t know what those data are among patients with HER2-positive breast cancer. I expect the number would be lower. I believe HER2-driven breast cancer is hormonally less sensitive. The same is true for chemotherapy. I don’t know of a good data set that I can use to tell the woman, “Your benefits from chemotherapy are X with HER2-driven breast cancer.”
Generally speaking, I multiply the risk by 1.5 in my head. So if an 8-mm, Grade II tumor — taking the HER2 status out of the equation — has about a 10 percent risk of systemic relapse, I usually multiply that by a 1.5 relative risk.
I’m starting at about a 15 percent risk of relapse, as far as I can possibly estimate. I don’t know exactly how much benefit hormonal therapy or chemotherapy alone will provide this patient.
So I tend to look at the data and say that I don’t see a lot of difference between an 8-mm tumor and a 10-mm tumor, which were allowed in BCIRG 006 and the HERA trial. Then I try to use the therapy to inhibit what is driving the cancer to minimize the risk part of the equation.
Track 17
![]() DR SLEDGE: In the two large North American trials, in which we used
doxorubicin and cyclophosphamide followed by paclitaxel and trastuzumab,
the congestive heart failure rates overall were somewhere in the three to four
percent range (Romond 2005). The questions that arise are, who are those
three to four percent of women and can we use that information to guide our
therapy?
DR SLEDGE: In the two large North American trials, in which we used
doxorubicin and cyclophosphamide followed by paclitaxel and trastuzumab,
the congestive heart failure rates overall were somewhere in the three to four
percent range (Romond 2005). The questions that arise are, who are those
three to four percent of women and can we use that information to guide our
therapy?
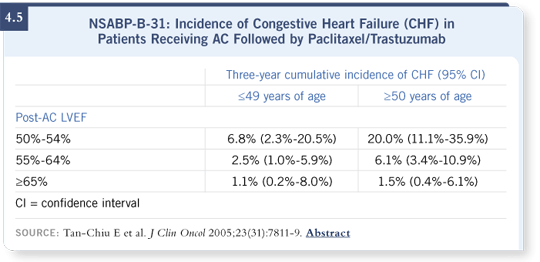
Today, we’re not sure. The NSABP database suggested that the women most likely to develop congestive heart failure were those who were older than age 50 and those with the lowest level of ejection fractions (50 to 55 percent) allowed to enroll in the trial. In NSABP-B-31, if you met those two criteria, you had roughly one chance in five of having a cardiac event (Tan-Chiu 2005; [4.5]), which is pretty significant.
I hasten to add that in NCCTG-N9831, an identical analysis was done and they did not come up with the same pattern (Perez 2005). When you run retrospective subset analyses of small subgroups in evolving trials, you can get very different results, although I don’t find it particularly surprising that older people would have more heart dysfunction.
I believe you can say, “You have a three to four percent chance of developing congestive heart failure. There is a reasonable likelihood that if you develop it, it will improve, but probably not all patients will improve. Also, those that improve may need to be on chronic medication for CHF, perhaps for the rest of their life.” That’s very definitely part of the trade-off, particularly for a patient with an 8-mm tumor.
![]() DR LOVE: What about TCH?
DR LOVE: What about TCH?
![]() DR SLEDGE: There, of course, we run into a different issue. Is TCH as good
as AC followed by TH? In the one trial we have, both regimens are better
than not receiving a trastuzumab-containing regimen and the regimens are
not statistically significantly different from each other with very early follow-up.
The trend in that trial certainly favors AC
DR SLEDGE: There, of course, we run into a different issue. Is TCH as good
as AC followed by TH? In the one trial we have, both regimens are better
than not receiving a trastuzumab-containing regimen and the regimens are
not statistically significantly different from each other with very early follow-up.
The trend in that trial certainly favors AC ![]() TH over TCH (Slamon
2005). So I personally tend to use TCH with patients for whom I have significant
concerns about cardiac toxicity.
TH over TCH (Slamon
2005). So I personally tend to use TCH with patients for whom I have significant
concerns about cardiac toxicity.
![]() DR LOVE: What would you say to the patient about the risk of cardiac
toxicity with TCH?
DR LOVE: What would you say to the patient about the risk of cardiac
toxicity with TCH?
![]() DR SLEDGE: It would be pretty trivial. A very small number of patients
developed congestive heart failure in that arm of the trial (Slamon 2005).
DR SLEDGE: It would be pretty trivial. A very small number of patients
developed congestive heart failure in that arm of the trial (Slamon 2005).
Track 19
![]() DR HARWIN: I started her on doxorubicin and cyclophosphamide with the
plan to treat her with trastuzumab and a taxane concomitantly, like the
patients in NCCTG-N9831. She has just started treatment.
DR HARWIN: I started her on doxorubicin and cyclophosphamide with the
plan to treat her with trastuzumab and a taxane concomitantly, like the
patients in NCCTG-N9831. She has just started treatment.
![]() DR LOVE: Can you follow up on your patient, Dr Hussein (Case 4)?
DR LOVE: Can you follow up on your patient, Dr Hussein (Case 4)?
![]() DR HUSSEIN: My patient qualified for BCIRG 006 because the trial allowed
the enrollment of patients with node-negative disease. She was randomly
assigned to receive docetaxel/carboplatin/trastuzumab (TCH). She received
six cycles and a whole year of trastuzumab. She did well, but her ejection
fraction dropped seven or eight points.
DR HUSSEIN: My patient qualified for BCIRG 006 because the trial allowed
the enrollment of patients with node-negative disease. She was randomly
assigned to receive docetaxel/carboplatin/trastuzumab (TCH). She received
six cycles and a whole year of trastuzumab. She did well, but her ejection
fraction dropped seven or eight points.
We stopped trastuzumab for a few weeks, and then we resumed it. She’s now at six months after finishing the study, and her ejection fraction is back to baseline.
![]() DR LOVE: How did she tolerate the TCH?
DR LOVE: How did she tolerate the TCH?
![]() DR HUSSEIN: She did very well. She’s an architect and was able to continue
working full time. The carboplatin/docetaxel caused a moderate degree of
fatigue, although her hemoglobin was normal. However, we got her through
the treatment.
DR HUSSEIN: She did very well. She’s an architect and was able to continue
working full time. The carboplatin/docetaxel caused a moderate degree of
fatigue, although her hemoglobin was normal. However, we got her through
the treatment.