
 |
||||||||

| Tracks 1-22 | ||||||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 5
![]() DR PEREZ: With a median follow-up of two years, the data demonstrated a
significant — not only statistical but clinical — improvement in disease-free,
distant disease-free and overall survival for the patients who were assigned to
receive concurrent paclitaxel/trastuzumab compared to those assigned to paclitaxel
alone.
DR PEREZ: With a median follow-up of two years, the data demonstrated a
significant — not only statistical but clinical — improvement in disease-free,
distant disease-free and overall survival for the patients who were assigned to
receive concurrent paclitaxel/trastuzumab compared to those assigned to paclitaxel
alone.
Another interesting aspect, which has been demonstrated in the other trastuzumab trials, is that the benefit of adding trastuzumab applied to all subgroups of patients with breast cancer. The benefit was irrespective of tumor size, nodal status or estrogen-receptor status (Romond 2005).
![]() DR LOVE: The distant disease-free survival curve from the combined analysis
was very striking (2.1). What do you think this means?
DR LOVE: The distant disease-free survival curve from the combined analysis
was very striking (2.1). What do you think this means?
![]() DR PEREZ: Dramatic — that’s the best word I can use — although not
unexpected in terms of showing a difference. What’s unexpected is the
magnitude of the difference. A clear benefit is irrefutable, even with this short
median follow-up. This is important for patients and physicians because many
times patients or physicians or regulatory agencies say, “In the adjuvant setting,
you need to wait 10 or 20 years to make a decision about therapy based on the
ultimate outcome.”
DR PEREZ: Dramatic — that’s the best word I can use — although not
unexpected in terms of showing a difference. What’s unexpected is the
magnitude of the difference. A clear benefit is irrefutable, even with this short
median follow-up. This is important for patients and physicians because many
times patients or physicians or regulatory agencies say, “In the adjuvant setting,
you need to wait 10 or 20 years to make a decision about therapy based on the
ultimate outcome.”
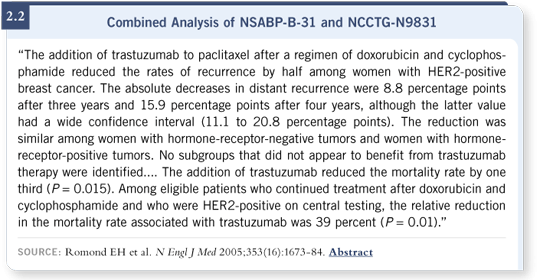
In these studies, even with the short median follow-up, the improvements in disease-free, overall and distant disease-free survival (2.2) are so dramatic that we cannot wait if we want to be ethical in our approach to patients. It is my belief that the difference in survival we have observed so far, which is already statistically significant, will increase in the future.
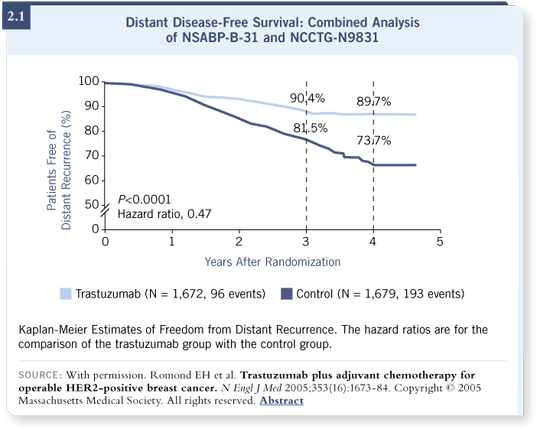
![]() DR LOVE: It was interesting that the distant disease-free survival curve looked
flat at around 90 percent for the trastuzumab arm (2.1).
DR LOVE: It was interesting that the distant disease-free survival curve looked
flat at around 90 percent for the trastuzumab arm (2.1).
![]() DR PEREZ: Yes. One of the things we are looking at is the hazard ratio over
time. It appears trastuzumab is working very quickly in terms of preventing
relapses in these patients with this aggressive type of breast cancer. Their
relapses are reaching a plateau, although we have to be careful because the
follow-up is short. However, in the control arm, the relapses continue occurring
at a higher rate.
DR PEREZ: Yes. One of the things we are looking at is the hazard ratio over
time. It appears trastuzumab is working very quickly in terms of preventing
relapses in these patients with this aggressive type of breast cancer. Their
relapses are reaching a plateau, although we have to be careful because the
follow-up is short. However, in the control arm, the relapses continue occurring
at a higher rate.
Track 7
![]() DR PEREZ: HERA is an important trial that is complementary to the studies
we conducted in the United States. The HERA investigators conducted a
three-arm trial in which trastuzumab was always administered sequentially to
chemotherapy and radiation therapy.
DR PEREZ: HERA is an important trial that is complementary to the studies
we conducted in the United States. The HERA investigators conducted a
three-arm trial in which trastuzumab was always administered sequentially to
chemotherapy and radiation therapy.
The three arms included chemotherapy/radiation therapy as per standard of care, another arm that allowed trastuzumab for one year and another arm that allowed trastuzumab for two years. In that trial, trastuzumab was administered once every three weeks (Piccart-Gebhart 2005), whereas in our studies, we used it once per week (Romond 2005).
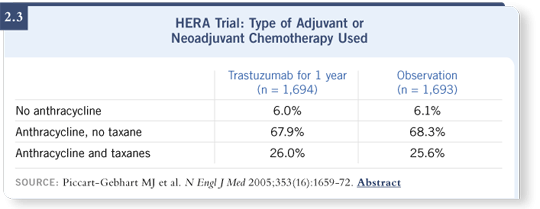
Some real issues related to HERA are important for clinical practice. Only about 26 percent of the patients in HERA received both anthracyclines and taxanes in the adjuvant setting (2.3). Therefore, the chemotherapy administered was quite different from the chemotherapy we administered in the US studies. About six percent of patients didn’t even receive anthracyclines (Piccart-Gebhart 2005; [2.3]). We need to continue following the curves, in terms of outcomes for the HERA trial.
I’m concerned that despite 32 percent of the patients enrolled in HERA having node-negative breast cancer, which would have led, in my opinion, to a better ultimate outcome for patients in the control arm, it appears that the patients in the control arm of the HERA trial did a little worse than the patients in the control arm of the US studies.
This may be a reflection of the differential outcomes for patients based on the country in which they were treated. It may also be that chemotherapy, especially the use of anthracyclines and taxanes, plays an important role in the setting of HER2-positive breast cancer. Seventy-four percent of the patients in HERA did not receive taxanes (Piccart-Gebhart 2005).
![]() DR LOVE: For those who did receive taxanes — and, of course, now we’re
getting into smaller numbers and subgroups — the effect of trastuzumab didn’t
seem to be as great.
DR LOVE: For those who did receive taxanes — and, of course, now we’re
getting into smaller numbers and subgroups — the effect of trastuzumab didn’t
seem to be as great.
![]() DR PEREZ: That’s a very good point, which has been missed by many people.
This is critically important, because NCCTG-N9831 did not show a dramatic
benefit with the use of trastuzumab in a sequential fashion following chemotherapy
(Perez 2005).
DR PEREZ: That’s a very good point, which has been missed by many people.
This is critically important, because NCCTG-N9831 did not show a dramatic
benefit with the use of trastuzumab in a sequential fashion following chemotherapy
(Perez 2005).
Our data are not that dissimilar from the data in the HERA study for the patients who received both anthracyclines and taxanes.
Track 8
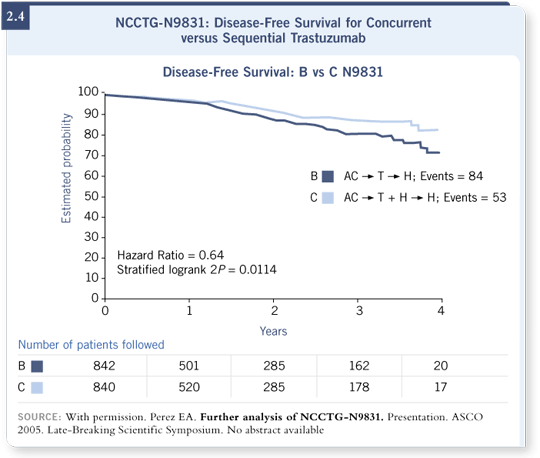
![]() DR PEREZ: Although we did not plan to conduct an analysis this early, we
have approximately 25 percent of the events required to be firm about the
statistical conclusions. We found, statistically, a better disease-free survival rate
for patients who received trastuzumab concurrently with paclitaxel compared
to those who received trastuzumab at the completion of paclitaxel (Perez
2005; [2.4]).
DR PEREZ: Although we did not plan to conduct an analysis this early, we
have approximately 25 percent of the events required to be firm about the
statistical conclusions. We found, statistically, a better disease-free survival rate
for patients who received trastuzumab concurrently with paclitaxel compared
to those who received trastuzumab at the completion of paclitaxel (Perez
2005; [2.4]).
Based on our data and this important trend, we recommend that patients receive concurrent trastuzumab with a taxane as adjuvant therapy for HER2-positive breast cancer.
![]() DR LOVE: How can you make this conclusion with only a quarter of the
events that you originally were targeting for this analysis?
DR LOVE: How can you make this conclusion with only a quarter of the
events that you originally were targeting for this analysis?
![]() DR PEREZ: We still reached a nominal p-value for the difference between
the concurrent and sequential arms. At this time, to be strict from the statistical
standpoint, we have a strong trend showing that concurrent is better than
sequential (Perez 2005; [2.4]). But again, it follows the data that exist both in
preclinical models and the metastatic setting.
DR PEREZ: We still reached a nominal p-value for the difference between
the concurrent and sequential arms. At this time, to be strict from the statistical
standpoint, we have a strong trend showing that concurrent is better than
sequential (Perez 2005; [2.4]). But again, it follows the data that exist both in
preclinical models and the metastatic setting.
Track 9
![]() DR PEREZ: BCIRG 006 evaluated AC followed by docetaxel, AC followed by
docetaxel with concurrent trastuzumab, and a third arm without an anthracycline
— specifically the TCH regimen, a combination of docetaxel, carboplatin
and trastuzumab. The results, as presented at the 2005 San Antonio Breast
Cancer Symposium, demonstrated a better outcome for the two trastuzumab-containing
arms compared to the control arm (Slamon 2005), which was similar to what we saw in the other studies.
I see an important trend in benefit with AC followed by docetaxel/trastuzumab
compared to TCH. Specifically, three-year disease-free survival was
86 percent for the patients who were assigned to AC followed by docetaxel/trastuzumab versus 80 percent for those assigned to TCH (Slamon 2005).
DR PEREZ: BCIRG 006 evaluated AC followed by docetaxel, AC followed by
docetaxel with concurrent trastuzumab, and a third arm without an anthracycline
— specifically the TCH regimen, a combination of docetaxel, carboplatin
and trastuzumab. The results, as presented at the 2005 San Antonio Breast
Cancer Symposium, demonstrated a better outcome for the two trastuzumab-containing
arms compared to the control arm (Slamon 2005), which was similar to what we saw in the other studies.
I see an important trend in benefit with AC followed by docetaxel/trastuzumab
compared to TCH. Specifically, three-year disease-free survival was
86 percent for the patients who were assigned to AC followed by docetaxel/trastuzumab versus 80 percent for those assigned to TCH (Slamon 2005).
Based on the results from BCIRG 006, we can say that no statistical difference exists between AC followed by docetaxel/trastuzumab and TCH. However, this trend should be taken into consideration when counseling patients. At this time, I would not favor TCH over an anthracycline/taxane/trastuzumab regimen.
Track 13
![]() DR PEREZ: The cardiac side effects associated with trastuzumab are minimal
compared to its efficacy in terms of disease-free and overall survival. At the
same time, we take cardiac risk very seriously, because we would like to
administer therapies that have no side effects. However, we cannot forget the
tremendous improvement in the lives of patients that we have achieved with
the use of trastuzumab.
DR PEREZ: The cardiac side effects associated with trastuzumab are minimal
compared to its efficacy in terms of disease-free and overall survival. At the
same time, we take cardiac risk very seriously, because we would like to
administer therapies that have no side effects. However, we cannot forget the
tremendous improvement in the lives of patients that we have achieved with
the use of trastuzumab.
I would like people to remember that it is not appropriate to do cross-study comparisons related to cardiac toxicity. The eligibility, based on age, and the definition of cardiac toxicity varied between the different trials. So it is dangerously incorrect to put tables together comparing the cardiac toxicity in BCIRG 006, HERA, NCCTG-N9831 and NSABP-B-31.
For NSABP-B-31, NCCTG-N9831 (Romond 2005) and HERA (Piccart-Gebhart 2005), we did not have an upper age limit for enrollment. In BCIRG 006, patients had to be 70 years old or younger (Slamon 2005). In the FinHER trial, patients had to be younger than 66 years of age (Joensuu 2006).
If we look at the enrollment according to left ventricular ejection fraction (LVEF) in NSABP-B-31 and NCCTG-N9831, the baseline LVEF had to be above the lower limit of normal (Romond 2005) or above 50 percent, whereas in HERA it had to be greater than 55 percent (Piccart-Gebhart 2005).
Additionally, let’s look at the definition of cardiac events. In the NCCTG-N9831 trial, NSABP-B-31 and BCIRG 006, if a patient developed one episode of decreased LVEF, that patient was counted in that group. In HERA, patients had to have a persistent decrease. So if the patient had only one decrease of LVEF, she was not counted in the group who developed decreases in LVEF.
Another important factor with HERA is that they divided patients with congestive heart failure into two groups — a mild and a severe group (Piccart-Gebhart 2005). When we reported our data from NCCTG-N9831, we combined the mild and severe cases. It’s going to be very important for us to compare apples to apples. We had a meeting with the cardiologists from HERA, NSABP-B-31 and BCIRG 006, and we’re trying to come up with common definitions of cardiac toxicity.
Track 14
![]() DR PEREZ: It’s about three percent. We’re finding out that this three percent
incidence appears to plateau. No differences appear between years two and
three in terms of the incidence of cardiac toxicity (Tan-Chiu 2005). It’s
specifically 3.5 percent in the AC followed by paclitaxel/trastuzumab arm of
NCCTG-N9831. I believe most of the cardiac toxicity events are appearing
quite early, but follow-up will be needed. The majority of these patients get
better quickly.
DR PEREZ: It’s about three percent. We’re finding out that this three percent
incidence appears to plateau. No differences appear between years two and
three in terms of the incidence of cardiac toxicity (Tan-Chiu 2005). It’s
specifically 3.5 percent in the AC followed by paclitaxel/trastuzumab arm of
NCCTG-N9831. I believe most of the cardiac toxicity events are appearing
quite early, but follow-up will be needed. The majority of these patients get
better quickly.
![]() DR LOVE: What would you say about cardiac safety to a patient who’s considering
TCH?
DR LOVE: What would you say about cardiac safety to a patient who’s considering
TCH?
![]() DR PEREZ: In BCIRG 006, no statistical difference in cardiac toxicity
appeared between TCH and AC followed by docetaxel/trastuzumab. I believe
in terms of cardiac toxicity, either regimen is pretty safe. I would not favor
one over another, based on the information presented by Dr Slamon at San
Antonio (Slamon 2005).
DR PEREZ: In BCIRG 006, no statistical difference in cardiac toxicity
appeared between TCH and AC followed by docetaxel/trastuzumab. I believe
in terms of cardiac toxicity, either regimen is pretty safe. I would not favor
one over another, based on the information presented by Dr Slamon at San
Antonio (Slamon 2005).