
 |
||||||||

| Tracks 1-18 | ||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 2
![]() DR HAYES: For older women, I believe the jury is out regarding the potential
benefits of chemotherapy. The issue has two components. One is whether
— for some mysterious reason — chemotherapy doesn’t work as well in older
women as in younger women. The second is whether the toxicities are greater
for older women and, therefore, the benefit-to-toxicity ratio is smaller.
DR HAYES: For older women, I believe the jury is out regarding the potential
benefits of chemotherapy. The issue has two components. One is whether
— for some mysterious reason — chemotherapy doesn’t work as well in older
women as in younger women. The second is whether the toxicities are greater
for older women and, therefore, the benefit-to-toxicity ratio is smaller.
If you take it to its extreme — and we didn’t put this in the editorial — another component is whether the number of life-years saved will be lower for older women and therefore not economically acceptable. An 80-year-old woman on average has another 10 years to live, but the number of life-years saved for her will be lower than for a 50-year-old woman for the same potential reduction in recurrences. Peter Ravdin has begun to build that into Adjuvant! Online. It’s not something we normally talk to patients about, but I believe it is part of the equation.
Track 3
![]() DR HAYES: CALGB-49907 (1.3) assumes that chemotherapy is beneficial.
It is not a trial of chemotherapy versus none. The question is whether in
this older age group one type of chemotherapy might be more acceptable by
being less toxic. Patients either receive one of the standard regimens — AC or
CMF — or capecitabine. A critical part of the study is to determine whether
capecitabine is a more acceptable regimen.
DR HAYES: CALGB-49907 (1.3) assumes that chemotherapy is beneficial.
It is not a trial of chemotherapy versus none. The question is whether in
this older age group one type of chemotherapy might be more acceptable by
being less toxic. Patients either receive one of the standard regimens — AC or
CMF — or capecitabine. A critical part of the study is to determine whether
capecitabine is a more acceptable regimen.
I believe this goes back to some of the biology. We know older women are more likely to have ER-positive cancers. I’m increasingly convinced that patients with ER-positive cancers are less sensitive to chemotherapy in general, not to a specific agent. Additionally, they are more sensitive to hormonal therapies compared to patients with ER-negative disease.
We’ve seen data from the individual cooperative groups, especially CALGB, and Don Berry (Berry 2006). We’ve also seen a hint of that in the last Overview, in that the proportional reduction among patients with ER-positive disease is less than among those with ER-negative disease (EBCTCG 2005). Most women who have breast cancer in their sixties and seventies have ER-positive disease, and this is one reason chemotherapy may be less effective in that group.
Track 8
![]() DR HAYES: Yes, we do. One reason is that we’re using the same regimen
across the board, which makes it safer for us. Second, I have not seen any evidence that dose-dense AC results in more cardiac toxicity than nondose-dense
AC. It’s been a concern, but so far, CALGB hasn’t seen it.
DR HAYES: Yes, we do. One reason is that we’re using the same regimen
across the board, which makes it safer for us. Second, I have not seen any evidence that dose-dense AC results in more cardiac toxicity than nondose-dense
AC. It’s been a concern, but so far, CALGB hasn’t seen it.
Third, although it does require growth factors, which increases the immediate costs of treatment and causes a few extra side effects, it’s completed so much faster. You cut a whole month off the treatment. If you use four cycles of dose-dense AC, you’re done in two months, which is terrific. At least, our patients say they like that.
Although the cost of the growth factors is elevated at the start, I’m convinced from the CALGB data that the lower number of hospital admissions required for neutropenia and fever outweigh it (Citron 2003).
Debate is ongoing about whether dose-dense therapy is better in terms of cancer outcomes. CALGB-9741 suggests it is. Another study published in the JNCI within the last year suggests that dose-dense FEC is not necessarily better (Venturini 2005).
Track 9
![]() DR HAYES: Genomic Health went to the NSABP and some other sources of
data and examined the records of patients with node-negative, ER-positive
disease who had received tamoxifen and had 10 or 15 years of follow-up. They
developed an algorithm and applied it to a second data set, NSABP-B-14, and
obtained exactly what they expected (Paik 2004).
DR HAYES: Genomic Health went to the NSABP and some other sources of
data and examined the records of patients with node-negative, ER-positive
disease who had received tamoxifen and had 10 or 15 years of follow-up. They
developed an algorithm and applied it to a second data set, NSABP-B-14, and
obtained exactly what they expected (Paik 2004).
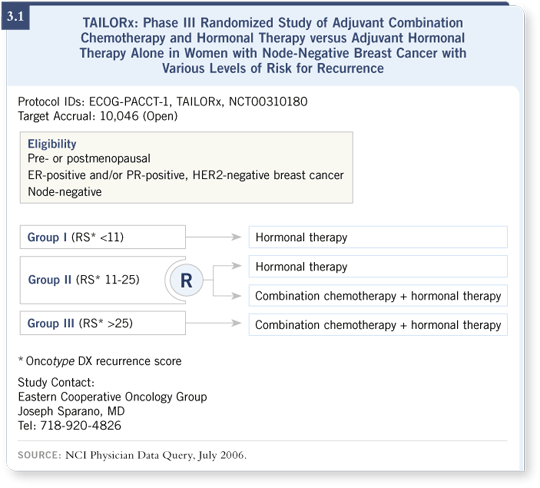
It’s very likely this test is accurate. However, I believe some caveats apply. These were older studies of older treatments, and the samples sat around for a while. For those reasons, the Intergroup is conducting a prospective study called TAILORx (3.1), in which women with ER-positive, node-negative disease will be profiled using the Oncotype DX assay.
If they have a low recurrence score, they will receive hormone therapy and be followed in a registry. If they have a high recurrence score, they will be invited to participate in chemotherapy trials within the Intergroup or be treated with chemotherapy off study and hormone therapy. If they have an intermediate recurrence score, for which we remain in enormous equipoise, they’ll be randomly assigned to hormone therapy alone or chemotherapy and hormone therapy.
Track 11
![]() DR HAYES: I don’t know the answer to that because HER2 factors very
heavily into Oncotype. One reason it might be useful is that I believe the technical aspects of HER2 analysis in this country are very poor. I believe a
lot of false-positive and false-negative results are presented, and that is true
whether you test by FISH or immunohistochemistry.
DR HAYES: I don’t know the answer to that because HER2 factors very
heavily into Oncotype. One reason it might be useful is that I believe the technical aspects of HER2 analysis in this country are very poor. I believe a
lot of false-positive and false-negative results are presented, and that is true
whether you test by FISH or immunohistochemistry.
Efforts are being made to standardize these tests. I expect you will see a lot of that in the next 12 months, both within the American Society of Clinical Oncology and the College of American Pathologists, because the stakes are high. Trastuzumab is an incredibly potent drug with an incredible price tag, both in terms of toxicities — the four to five percent risk of cardiac dysfunction in the short run and the potential long-term toxicities — and the actual dollar costs.
We need to standardize HER2 testing, and we will. The Oncotype DX assay might be a useful way to look at HER2 expression. Certainly it’s built in.
Track 12
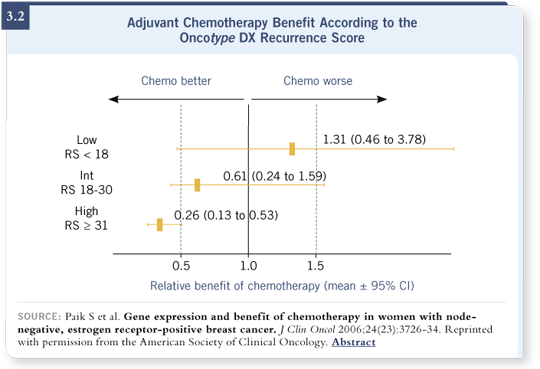
![]() DR HAYES: Not quite. I’m pretty comfortable with the accuracy of the
Oncotype DX assay relative to the benefit from tamoxifen, meaning that if you
have ER-positive, node-negative disease and you receive tamoxifen, I believe
the Oncotype DX assay provides an accurate outcome score. The predictive
value of the Oncotype DX score for chemotherapy benefit, however, is based
on a very small group of patients, and the confidence limits are enormous
(3.2).
DR HAYES: Not quite. I’m pretty comfortable with the accuracy of the
Oncotype DX assay relative to the benefit from tamoxifen, meaning that if you
have ER-positive, node-negative disease and you receive tamoxifen, I believe
the Oncotype DX assay provides an accurate outcome score. The predictive
value of the Oncotype DX score for chemotherapy benefit, however, is based
on a very small group of patients, and the confidence limits are enormous
(3.2).
The patients with a high recurrence score tend to have lower ER levels. They’re not ER-negative but show lower ER and higher HER2 levels. That’s the group I would have guessed to have a higher proportional reduction from chemotherapy, and Oncotype DX suggests that’s true. I expect the true reduction has yet to be precisely estimated, because the confidence limits are so large. We’re talking of only about 200 patients from NSABP-B-20 (Paik 2006).
Tracks 13-14
![]() DR HAYES: I believe an important issue, which has been lost, is that all of
the aromatase inhibitor studies enrolled women who were postmenopausal by
virtue of not having a period for at least a year prior to enrollment. We have
estrogen ablation studies ongoing for premenopausal women, such as SOFT, TEXT and PERCHE. We don’t know the answers from those studies yet.
DR HAYES: I believe an important issue, which has been lost, is that all of
the aromatase inhibitor studies enrolled women who were postmenopausal by
virtue of not having a period for at least a year prior to enrollment. We have
estrogen ablation studies ongoing for premenopausal women, such as SOFT, TEXT and PERCHE. We don’t know the answers from those studies yet.
I believe estrogen ablation is a more effective therapy than a SERM, but I also believe it’s more toxic. I’m very supportive of those trials. We have enrolled 11 patients on SOFT. They’re important studies, almost as much for the toxicity as for the outcomes.
The ovaries can go to sleep and wake back up again. Ian Smith at the Royal Marsden and I discussed this at a meeting. He went back and retrospectively reviewed his institution’s experience with women who had received chemotherapy, became amenorrheic and were then placed on an aromatase inhibitor.
About one quarter of those patients had their ovarian function reemerge, either by virtue of developing menses or by having their estrogen levels increased (Smith 2006).