
 |
| Tracks 1-9 | ||||||||||||||||||||
|
Editor’s note: A few weeks subsequent to the audio recording of the November 2006 interviews with Drs Steingart and Slamon and the Tumor Panel Discussion, Dr Slamon presented an updated analysis of BCIRG 006 at the San Antonio Breast Cancer Symposium. Dr Burstein was interviewed immediately after this presentation to discuss the findings.
Select Excerpts from the Interview
Track 2
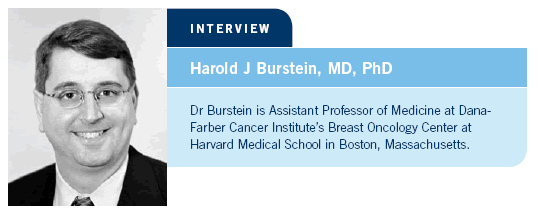
![]() DR LOVE: First, by way of background, can you summarize the BCIRG
006 data originally reported at the San Antonio Breast Cancer Symposium
in 2005 (Slamon 2005)?
DR LOVE: First, by way of background, can you summarize the BCIRG
006 data originally reported at the San Antonio Breast Cancer Symposium
in 2005 (Slamon 2005)?
![]() DR BURSTEIN: BCIRG 006 was one of four or five major randomized trials
evaluating adjuvant trastuzumab combined with chemotherapy for patients
with HER2-positive breast cancer. In this trial, approximately 3,200 patients
were randomly assigned to doxorubicin/cyclophosphamide followed by
docetaxel (AC
DR BURSTEIN: BCIRG 006 was one of four or five major randomized trials
evaluating adjuvant trastuzumab combined with chemotherapy for patients
with HER2-positive breast cancer. In this trial, approximately 3,200 patients
were randomly assigned to doxorubicin/cyclophosphamide followed by
docetaxel (AC![]() T) or AC followed by docetaxel with trastuzumab (AC
T) or AC followed by docetaxel with trastuzumab (AC![]() TH) or docetaxel, carboplatin and trastuzumab (TCH). Approximately
30 percent of the patients had node-negative disease, and all of the tumors
were HER2-positive by FISH.
TH) or docetaxel, carboplatin and trastuzumab (TCH). Approximately
30 percent of the patients had node-negative disease, and all of the tumors
were HER2-positive by FISH.
The BCIRG trial differed from some of the others in that it had a nonanthracycline-containing arm. The rationale for this arm was twofold. One reason was to determine whether cardiotoxicity, which had been seen in the metastatic setting, could be avoided during adjuvant therapy with trastuzumab. A second reason was to study one of the combinations of chemotherapy that had been shown in the laboratory to have dramatic synergy with trastuzumab, and they selected docetaxel with carboplatin.
When the data were originally presented at the San Antonio Breast Cancer
Symposium in 2005, we saw that both the trastuzumab-based arms,
AC![]() TH and TCH, were superior to the nontrastuzumab-containing arm,
AC
TH and TCH, were superior to the nontrastuzumab-containing arm,
AC![]() T. That fit with the results from the other four major adjuvant trials
(Joensuu 2005; Piccart-Gebhart 2005; Romond 2005).
T. That fit with the results from the other four major adjuvant trials
(Joensuu 2005; Piccart-Gebhart 2005; Romond 2005).
The second finding was that the incidence of cardiac toxicity seemed to be
lower in the TCH arm versus AC![]() TH. Although the differences were not
statistically significant, approximately two and a half percent of patients in
the AC
TH. Although the differences were not
statistically significant, approximately two and a half percent of patients in
the AC![]() TH arm versus about one percent in the TCH arm experienced
symptoms consistent with congestive heart failure. Also a greater fraction of
patients receiving AC
TH arm versus about one percent in the TCH arm experienced
symptoms consistent with congestive heart failure. Also a greater fraction of
patients receiving AC![]() TH had asymptomatic changes in LVEF (Slamon
2005).
TH had asymptomatic changes in LVEF (Slamon
2005).
A third finding, which was not statistically significant but was visually
apparent when examining the Kaplan-Meier curves, was that AC![]() TH was
superior to TCH in terms of efficacy. The difference was approximately four
percent (Slamon 2005).
TH was
superior to TCH in terms of efficacy. The difference was approximately four
percent (Slamon 2005).
For those reasons, I and many other clinicians continued to favor an anthracycline- and taxane-based trastuzumab regimen, akin to what NSABP-B-31 and the North American Intergroup study (NCCTG-N9831) had shown. My preference was for the Intergroup regimen — four cycles of AC followed by 12 weeks of paclitaxel with trastuzumab. Having participated in the trial, we had experience with and extensive, well-documented safety information for that regimen.
Track 3
![]() DR LOVE: What did the second interim analysis of BCIRG 006 show as
presented yesterday by Dr Slamon (Slamon 2006)?
DR LOVE: What did the second interim analysis of BCIRG 006 show as
presented yesterday by Dr Slamon (Slamon 2006)?
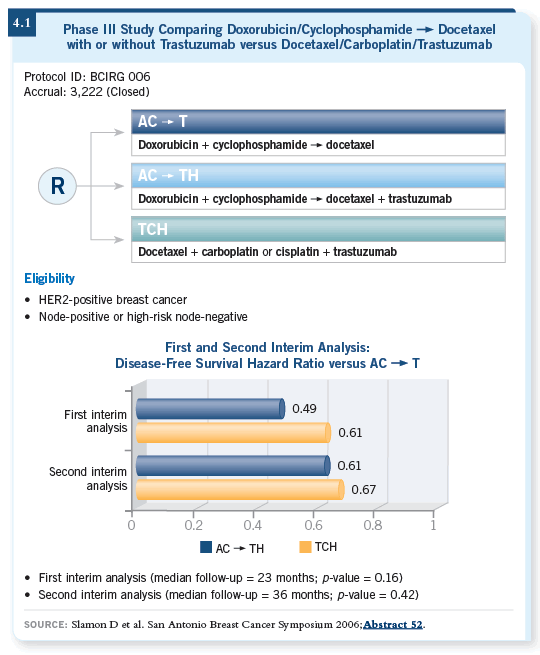
![]() DR BURSTEIN: With approximately 12 more months of follow-up, the data
had changed a bit. Whereas the basic findings were the same — that is, the
trastuzumab-containing arms continued to outperform the nontrastuzumab-containing
arm — the efficacy differences between AC
DR BURSTEIN: With approximately 12 more months of follow-up, the data
had changed a bit. Whereas the basic findings were the same — that is, the
trastuzumab-containing arms continued to outperform the nontrastuzumab-containing
arm — the efficacy differences between AC![]() TH and TCH
became less apparent (4.1, 4.2). In fact, the curves now track closely together,
with only about a one percent difference separating them (Slamon 2006).
TH and TCH
became less apparent (4.1, 4.2). In fact, the curves now track closely together,
with only about a one percent difference separating them (Slamon 2006).
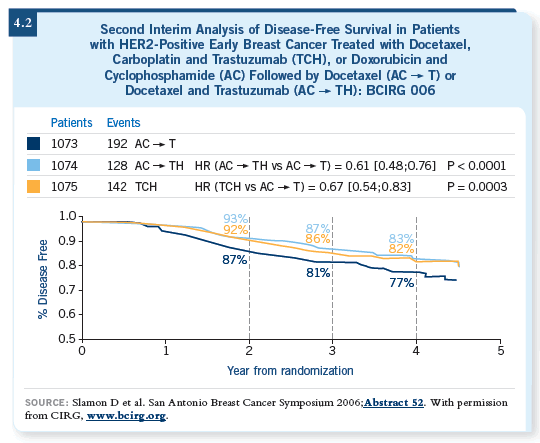
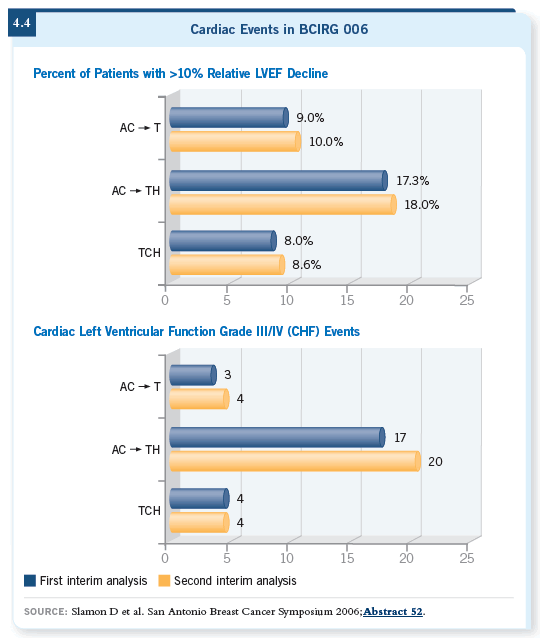
The updated analyses of cardiac function continue to show a lower risk of symptomatic congestive heart failure with the TCH regimen (Slamon 2006; [4.3, 4.4]). Also, with the longer follow-up, four cases of leukemia emerged among the roughly 2,100 women who received one of the anthracycline-based regimens. That’s a low percentage in absolute terms, but it’s consistent with prior reports of anthracycline-based regimens. In the 1,100 or so women who received the TCH regimen, no cases of leukemia have been reported (4.3).
I believe the BCIRG 006 data stand out as one of the highlights of the 2006 San Antonio Breast Cancer Symposium and will result in clinicians considering the TCH regimen much more often as an option for patients with HER2-positive, early-stage breast cancer. It seems to be as efficacious as the anthracycline-based regimens and to have a better toxicity profile with respect to certain rare, but serious, late complications (Slamon 2005).
Track 4
![]() DR LOVE: Would you summarize the TOPO II data from BCIRG 006?
DR LOVE: Would you summarize the TOPO II data from BCIRG 006?
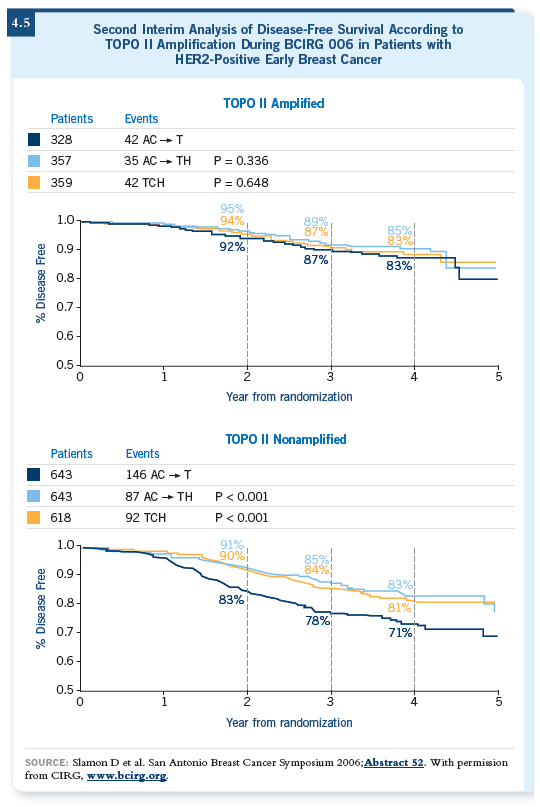
![]() DR BURSTEIN: The TOPO II issue, which has been discussed a lot since the
BCIRG 006 data were presented in 2005, looks less relevant now with the
2006 data (Press 2005; Slamon 2006; [4.5, 4.6]).
DR BURSTEIN: The TOPO II issue, which has been discussed a lot since the
BCIRG 006 data were presented in 2005, looks less relevant now with the
2006 data (Press 2005; Slamon 2006; [4.5, 4.6]).
The TOPO II gene is on human chromosome 17, not too far from the HER2/neu locus. In some cases of acquired HER2 gene amplification, you also have amplification of the TOPO II locus. TOPO II is a target of anthracyclines, and many people have suggested that TOPO II overexpression particularly identifies tumors that benefit from anthracyclines.
In the preliminary work from the BCIRG 006 trial that Dennis Slamon and
Mike Press reported at the San Antonio meeting in 2005, they suggested
that in TOPO II overexpressors, the anthracycline/ trastuzumab (AC![]() TH)
arm was superior to the nonanthracycline/trastuzumab (TCH) arm. For the
majority of tumors in which the TOPO II is not amplified, however, TCH was
more or less equivalent to AC
TH)
arm was superior to the nonanthracycline/trastuzumab (TCH) arm. For the
majority of tumors in which the TOPO II is not amplified, however, TCH was
more or less equivalent to AC![]() TH (Press 2005). If in the aggregate they’re
the same, it washes out the effects of the TOPO II test question. I believe if clinicians decide that they can use a nonanthracycline/
TH (Press 2005). If in the aggregate they’re
the same, it washes out the effects of the TOPO II test question. I believe if clinicians decide that they can use a nonanthracycline/
trastuzumab-based
regimen, it doesn’t matter whether they perform the TOPO II testing.
In the 35 percent of cases in which the tumor was both HER2-positive and TOPO II-positive, the curves all track similarly, which is a puzzle (Slamon 2006). I’m not sure I have a brilliant explanation for that. One thought would be that anthracyclines are important if the tumor is TOPO II-positive and perhaps those tumors have a greater resistance to trastuzumab, such that we don’t see a huge additional benefit.
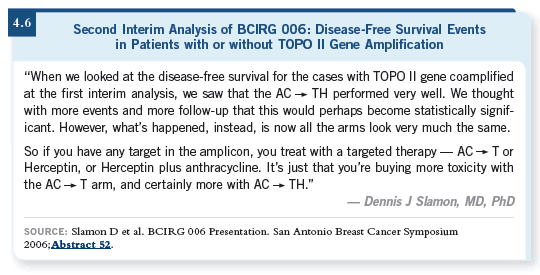
One thing to remember is that curves are not etched in marble, as Slamon’s additional one-year follow-up data illustrate. Data evolve over time. The data presented in 2005 had a median follow-up of approximately two to two and a half years. Although I don’t expect the fundamental conclusions will change in any major way, it is important to realize that as time goes by, you will see some evolution of the curves and in this trial that may be clinically relevant. Also, any time you have subsets of subsets and you’re talking about a lower number of cases, then the curves are less stable.
![]() DR LOVE: What about the curves for the TOPO II-nonamplified tumors?
DR LOVE: What about the curves for the TOPO II-nonamplified tumors?
![]() DR BURSTEIN: For those patients, the trastuzumab-based arms are superior to
the nontrastuzumab arm. Visually, there’s less difference between the two trastuzumab-based arms than there seemed to be a year ago (Slamon 2006; [4.5, 4.6]).
DR BURSTEIN: For those patients, the trastuzumab-based arms are superior to
the nontrastuzumab arm. Visually, there’s less difference between the two trastuzumab-based arms than there seemed to be a year ago (Slamon 2006; [4.5, 4.6]).
![]() DR LOVE: I’ve talked to a number of clinicians who in a few difficult cases
sent specimens to Mike Press at UCLA to test TOPO II this past year, but
now, based on the new data, they don’t plan to do that anymore. Does that
seem logical?
DR LOVE: I’ve talked to a number of clinicians who in a few difficult cases
sent specimens to Mike Press at UCLA to test TOPO II this past year, but
now, based on the new data, they don’t plan to do that anymore. Does that
seem logical?
![]() DR BURSTEIN: I wasn’t certain it was logical to begin with because the data
were preliminary with short follow-up and reflected subsets of patients in a
three-arm study, which is always dodgy. At this point, I believe there’s even less rationale for testing. I assume clinicians will either continue to use
AC
DR BURSTEIN: I wasn’t certain it was logical to begin with because the data
were preliminary with short follow-up and reflected subsets of patients in a
three-arm study, which is always dodgy. At this point, I believe there’s even less rationale for testing. I assume clinicians will either continue to use
AC![]() TH, because they’ll say that’s what they’ve always used and it’s effective,
or they’ll increasingly switch to TCH. In either case, you don’t need TOPO II
testing to help you.
TH, because they’ll say that’s what they’ve always used and it’s effective,
or they’ll increasingly switch to TCH. In either case, you don’t need TOPO II
testing to help you.
Track 5
![]() DR LOVE: What do you see as the future role for anthracyclines?
DR LOVE: What do you see as the future role for anthracyclines?
![]() DR BURSTEIN: In trying to determine the best chemotherapy foundation for
trastuzumab in the adjuvant setting, we have compelling data for several strategies.
Using TCH looks more viable than it did a year ago. In the BCIRG 006
trial, TCH seems to be as efficacious as AC
DR BURSTEIN: In trying to determine the best chemotherapy foundation for
trastuzumab in the adjuvant setting, we have compelling data for several strategies.
Using TCH looks more viable than it did a year ago. In the BCIRG 006
trial, TCH seems to be as efficacious as AC![]() TH (4.3).
TH (4.3).
A lot of retrospective analyses, including updates of multiple trials presented at the 2006 San Antonio meeting, strongly suggest that the tumors that most benefit from anthracycline-based therapy are those that are HER2 amplified or TOPO II amplified (Gunnarsdottir 2006; Johnson 2006; O’Malley 2006). However, we now have a whole different treatment paradigm for them. The patients with HER2-positive disease all receive trastuzumab-based therapy, and in patients with HER2-negative disease, it’s difficult to see much benefit from anthracycline-based regimens over nonanthracycline-based regimens.
Anthracyclines have been useful for many women for a long time, and they probably have contributed to much better outcomes for these women. However, because we increasingly have subsets of breast cancer for which we have different treatment programs — for example, patients with HER2-positive disease receive trastuzumab and patients with HER2-negative disease might not need as much chemotherapy or might need different flavors of chemotherapy — we increasingly have the flexibility of offering nonanthracycline-based regimens.
Many nonanthracycline-based regimens are available. In addition to CMF, we have taxane-based regimens, such as the docetaxel/cyclophosphamide regimen that the US Oncology group has put forward ( Jones 2006), and others will continue to emerge. I believe this is something we should continue to explore vigorously in clinical trials because it might spare our patients some toxicity.
![]() DR LOVE: To what extent are you using nonanthracycline-containing
regimens for your patients with HER2-negative breast cancer?
DR LOVE: To what extent are you using nonanthracycline-containing
regimens for your patients with HER2-negative breast cancer?
![]() DR BURSTEIN: I still frequently use four cycles of AC or AC followed by a
taxane for non-HER2-positive disease. We have a lot of data suggesting that
dose-dense AC followed by paclitaxel, as used in CALGB-9741, is a highly
effective regimen for breast cancer, particularly for patients with ER-negative
disease (Citron 2003). I have also used CMF in the adjuvant setting.
DR BURSTEIN: I still frequently use four cycles of AC or AC followed by a
taxane for non-HER2-positive disease. We have a lot of data suggesting that
dose-dense AC followed by paclitaxel, as used in CALGB-9741, is a highly
effective regimen for breast cancer, particularly for patients with ER-negative
disease (Citron 2003). I have also used CMF in the adjuvant setting.
Studies have examined AC followed by paclitaxel on an every three-week schedule and suggest that it is an inferior regimen to other options (Burnell 2006). I believe every three-week anthracyclines are on their way out. Whether dose-densifying anthracycline-based regimens continues to be superior to nonanthracycline options such as CMF or TC is something that we need to explore.
Track 6
![]() DR LOVE: Dr Slamon feels that nonanthracycline-containing regimens,
such as TCH, have a significantly better cardiac safety profile than the
anthracycline-based regimens. Do you agree?
DR LOVE: Dr Slamon feels that nonanthracycline-containing regimens,
such as TCH, have a significantly better cardiac safety profile than the
anthracycline-based regimens. Do you agree?
![]() DR BURSTEIN: I only have the data Dr Slamon has presented, and the differences
in the absolute incidence of cardiotoxicity are very small, approximately
a percentage point (Slamon 2006). In addition, a slightly greater percentage of
patients have an asymptomatic decline in ejection fraction with AC
DR BURSTEIN: I only have the data Dr Slamon has presented, and the differences
in the absolute incidence of cardiotoxicity are very small, approximately
a percentage point (Slamon 2006). In addition, a slightly greater percentage of
patients have an asymptomatic decline in ejection fraction with AC![]() TH, the
significance of which is unknown.
TH, the
significance of which is unknown.
I believe that the ultimate efficacy of the regimen is what should mostly dictate which treatment to use. If two regimens are equally efficacious, then you focus on convenience, side-effect profiles and late — but serious — consequences. With respect to those choices, TCH looks better today than it did a year ago because it’s now equally efficacious and seems to have some advantages with respect to safety (Slamon 2006; [4.4]).
![]() DR LOVE: Have you treated any patients with HER2-positive breast cancer
with a nonanthracycline-containing regimen in the clinical setting?
DR LOVE: Have you treated any patients with HER2-positive breast cancer
with a nonanthracycline-containing regimen in the clinical setting?
![]() DR BURSTEIN: Since the data were presented at ASCO, I have not because
approximately 9,000 of the 10,000 women treated in the adjuvant trastuzumab
randomized studies received anthracyclines and I believe that before everybody
walks away from anthracyclines, we need ongoing follow-up of all these trials
(Perez 2005; Joensuu 2005; Piccart-Gebhart 2005; Romond 2005; Slamon
2006; Smith 2007). I do believe that clinicians will use TCH more often.
DR BURSTEIN: Since the data were presented at ASCO, I have not because
approximately 9,000 of the 10,000 women treated in the adjuvant trastuzumab
randomized studies received anthracyclines and I believe that before everybody
walks away from anthracyclines, we need ongoing follow-up of all these trials
(Perez 2005; Joensuu 2005; Piccart-Gebhart 2005; Romond 2005; Slamon
2006; Smith 2007). I do believe that clinicians will use TCH more often.
We have been considering strategies for treating patients with HER2-positive breast cancer with nonanthracycline regimens. In early 2007, we’ll be inaugurating a feasibility study for patients with Stage I breast cancer in which patients will receive 12 weeks of paclitaxel with trastuzumab. We have relatively few data on the benefits of trastuzumab for these women at lower risk. We believe that 12 weeks of a chemotherapy agent that’s reasonably well tolerated, such as weekly paclitaxel, might be an effective treatment.
Track 7
![]() DR LOVE: Would you discuss the data from the Phase II trial of bevacizumab
and trastuzumab in women with advanced HER2-positive breast
cancer?
DR LOVE: Would you discuss the data from the Phase II trial of bevacizumab
and trastuzumab in women with advanced HER2-positive breast
cancer?
![]() DR BURSTEIN: There is no reason to believe bevacizumab would work only
in HER2-negative breast cancer. However, we have surprisingly few data for
bevacizumab in HER2-positive breast cancer. That was a part of the motivation
for Pegram’s study at UCLA, and the other part was an interest in determining
whether it would be safe and effective to pair these two humanized monoclonal
antibodies — trastuzumab and bevacizumab — with one another.
DR BURSTEIN: There is no reason to believe bevacizumab would work only
in HER2-negative breast cancer. However, we have surprisingly few data for
bevacizumab in HER2-positive breast cancer. That was a part of the motivation
for Pegram’s study at UCLA, and the other part was an interest in determining
whether it would be safe and effective to pair these two humanized monoclonal
antibodies — trastuzumab and bevacizumab — with one another.
When Dr Pegram presented the Phase I data for approximately a dozen patients, he showed that there did not seem to be pharmacokinetic interactions between bevacizumab and trastuzumab (Pegram 2004). He followed up on this by showing preliminary results from the open-label Phase II trial, where chemotherapy-naïve patients with HER2-positive breast cancer receive trastuzumab and bevacizumab in the metastatic setting (Pegram 2006).
Although the data presented were somewhat premature because they are still accruing patients, the response rate was dramatic — 54 percent — in the 37 assessable patients. That’s interesting because it suggests that a nonchemotherapy regimen with potent biological agents could have substantial activity.
One concern is the safety of this combination, and it looks feasible. One case of symptomatic heart failure in these 37 patients was reported, but it’s not clear if that’s an artifact of single-agent trastuzumab, which can rarely cause congestive heart failure, or if there’s some other signal.
About another dozen patients had asymptomatic changes in LVEF of unclear
significance. In all, about 35 percent of the patients had asymptomatic changes
in LVEF, which is a high number. It’s higher than we saw in the AC![]() TH
arm in the adjuvant trial. We need to determine in the metastatic setting
whether that has long-term consequences.
TH
arm in the adjuvant trial. We need to determine in the metastatic setting
whether that has long-term consequences.
This trial suggests that bevacizumab and trastuzumab can be combined safely, and it invites ongoing research with this doublet to see if that’s a better way to treat HER2-positive disease. It may also generate interest in adjuvant trials. I suspect the next generation of adjuvant trials from some of the cooperative groups will evaluate chemotherapy with trastuzumab, with or without bevacizumab, in patients with HER2-positive breast cancer.