
 |

| Tracks 1-18 | ||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 2-3
![]() DR LOVE: Can you discuss the impact of “holding” trastuzumab due to
anthracycline-associated declines in LVEF?
DR LOVE: Can you discuss the impact of “holding” trastuzumab due to
anthracycline-associated declines in LVEF?
![]() DR SLAMON: In the adjuvant trastuzumab trials, we frequently saw patients
with HER2-positive disease who were treated with AC but never received the taxane/trastuzumab therapy because they had declines in their LVEF, yet those
are not scored as a toxicity.
DR SLAMON: In the adjuvant trastuzumab trials, we frequently saw patients
with HER2-positive disease who were treated with AC but never received the taxane/trastuzumab therapy because they had declines in their LVEF, yet those
are not scored as a toxicity.
It’s a greater negative when that happens than when other things happen because now you’re denying a potentially active drug to a woman who might benefit from it because you forced the agenda with the anthracycline. That happens between three and five percent of the time in all the studies that were examined, and it’s more frequent in older patients. The problem is that once physicians see the LVEF drop, they’re reluctant to go ahead and take the risk.
![]() DR LOVE: What do we know about the long-term effects and reversibility of
cardiotoxicity associated with trastuzumab and anthracyclines?
DR LOVE: What do we know about the long-term effects and reversibility of
cardiotoxicity associated with trastuzumab and anthracyclines?
![]() DR SLAMON: Since the release of the data presented at ASCO in 2005, I
believe oncologists are becoming increasingly aware that the cardiac toxicities
might continue for longer than previously believed. The assumption had been
that once we stop trastuzumab, the cardiac problems reversed in a matter of a
couple of weeks or months. However, the data — at least the BCIRG 006 data
— show that it is longer lasting. We now know that a year and a half later,
those subclinical LVEF declines seem to be maintained at some level.
DR SLAMON: Since the release of the data presented at ASCO in 2005, I
believe oncologists are becoming increasingly aware that the cardiac toxicities
might continue for longer than previously believed. The assumption had been
that once we stop trastuzumab, the cardiac problems reversed in a matter of a
couple of weeks or months. However, the data — at least the BCIRG 006 data
— show that it is longer lasting. We now know that a year and a half later,
those subclinical LVEF declines seem to be maintained at some level.
We previously thought that the patients with clinical congestive heart failure improved with treatment. However, at least two thirds of them require continued treatment. That means you can treat their congestive heart failure, but it doesn’t mean you’ve made that heart better. I believe these definitions must be more carefully stated when some of these data are presented.
We thought the cardiac problems did not occur until after a certain large cumulative dose of anthracyclines. However, as we’re carefully measuring the ejection fractions in the trastuzumab trials, we’re seeing them much earlier than anyone previously thought.
At doses of 150 mg/m2 and higher, you begin to see this issue “raise its head.” Those doses were always thought to have a safe cardiac profile. As we examine this more carefully, we are seeing that left ventricular dysfunction is not just the noise of the MUGA scan or the ECHO but real left ventricular dysfunction at very low doses of doxorubicin. It is a potential long-term problem.
Track 5
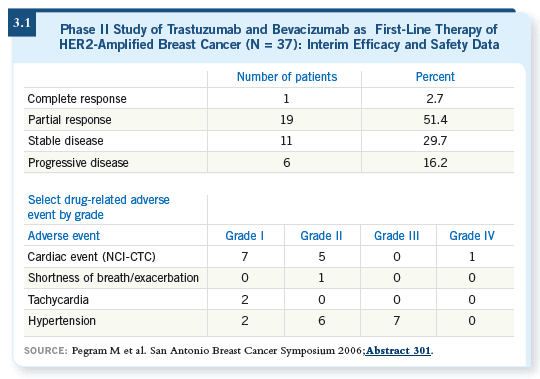
![]() DR LOVE: Data will be presented at the 2006 San Antonio Breast Cancer
Symposium from the trial combining bevacizumab and trastuzumab in
the adjuvant setting. Can you discuss that study (Pegram 2006; [3.1])?
DR LOVE: Data will be presented at the 2006 San Antonio Breast Cancer
Symposium from the trial combining bevacizumab and trastuzumab in
the adjuvant setting. Can you discuss that study (Pegram 2006; [3.1])?
![]() DR SLAMON: Mark Pegram will be presenting that trial (Pegram 2006), and
we’re excited about the data we’re seeing with bevacizumab and trastuzumab.
The challenge will be how to administer that combination safely.
DR SLAMON: Mark Pegram will be presenting that trial (Pegram 2006), and
we’re excited about the data we’re seeing with bevacizumab and trastuzumab.
The challenge will be how to administer that combination safely.
We’re guaranteed to cause hypertension in 15 to 20 percent of the women on standard-dose bevacizumab, and most of us believe we are already at the wall regarding cardiac dysfunction with the combination of trastuzumab and doxorubicin. So the question is, if we administer bevacizumab on that same backbone, will we offset any increased benefits by increasing the toxicity? That’s a concern we have at this point.
![]() DR LOVE: Have any cardiac studies evaluated the combination of bevacizumab,
trastuzumab and doxorubicin?
DR LOVE: Have any cardiac studies evaluated the combination of bevacizumab,
trastuzumab and doxorubicin?
![]() DR SLAMON: Studies have been conducted with bevacizumab and doxorubicin,
and there’s a cardiac signal there, but it’s weak, but we all know about
the signal seen when combining trastuzumab and doxorubicin.
DR SLAMON: Studies have been conducted with bevacizumab and doxorubicin,
and there’s a cardiac signal there, but it’s weak, but we all know about
the signal seen when combining trastuzumab and doxorubicin.
The question is whether we will potentiate that significantly when we begin with the cardiac insult of doxorubicin and increase LVEF in some percentage of the patients by causing the hypertension and then blocking the HER2 protective effect on the heart. We won’t know that until we see data from a clinical trial.
![]() DR LOVE: Do we have any data on the cardiac effects of just bevacizumab
with trastuzumab?
DR LOVE: Do we have any data on the cardiac effects of just bevacizumab
with trastuzumab?
![]() DR SLAMON: They’re being generated. In our pilot trial of bevacizumab and
trastuzumab, we saw an increased incidence of cardiac dysfunction. In the first
29 cases examined, there were two clinical congestive failures, one of which
required ICU hospitalization and pressers. Also, we’re seeing a large number
of patients with Class I or II dysfunction. It’s a first-line metastatic trial, and
these patients have received doxorubicin in the past.
DR SLAMON: They’re being generated. In our pilot trial of bevacizumab and
trastuzumab, we saw an increased incidence of cardiac dysfunction. In the first
29 cases examined, there were two clinical congestive failures, one of which
required ICU hospitalization and pressers. Also, we’re seeing a large number
of patients with Class I or II dysfunction. It’s a first-line metastatic trial, and
these patients have received doxorubicin in the past.
We believe just the two biologic agents can be administered safely. The question in the adjuvant setting will be, what happens if you add doxorubicin? Docetaxel/carboplatin/trastuzumab (TCH) is in play because it gives us a noncardiotoxic regimen if the efficacy of bevacizumab and trastuzumab is significant and warrants that that should be used next.
We’re considering a study that evaluates some variation of AC![]() TH as the
new standard control arm versus AC
TH as the
new standard control arm versus AC![]() THB versus TCHB, if we’re not using
TOPO II amplification.
THB versus TCHB, if we’re not using
TOPO II amplification.
If we use TOPO II amplification, an alternative design would be that patients
who have TOPO II-amplified disease would receive AC![]() TH versus
AC
TH versus
AC![]() THB, and patients who have non-TOPO II-amplified disease would
receive TCH versus TCHB. That’s assuming the BCIRG 006 data continue
to show that the benefit accrued with an anthracycline is, for the large part,
restricted to the TOPO II data (Press 2005; Slamon 2005). Either of these
study designs would answer whether you can safely administer trastuzumab
and bevacizumab together.
THB, and patients who have non-TOPO II-amplified disease would
receive TCH versus TCHB. That’s assuming the BCIRG 006 data continue
to show that the benefit accrued with an anthracycline is, for the large part,
restricted to the TOPO II data (Press 2005; Slamon 2005). Either of these
study designs would answer whether you can safely administer trastuzumab
and bevacizumab together.
![]() DR LOVE: What’s the next step?
DR LOVE: What’s the next step?
![]() DR SLAMON: The NSABP approached the BCIRG about collaborating on the
next generation of trastuzumab trials. We have an enormous amount of respect
for the NSABP, so we are considering it, assuming we can arrive at a common
design that we both feel comfortable with. A major meeting of the two groups
will be held at the San Antonio meeting to discuss this.
DR SLAMON: The NSABP approached the BCIRG about collaborating on the
next generation of trastuzumab trials. We have an enormous amount of respect
for the NSABP, so we are considering it, assuming we can arrive at a common
design that we both feel comfortable with. A major meeting of the two groups
will be held at the San Antonio meeting to discuss this.
Track 7
![]() DR LOVE: How do you think the efficacy data for adjuvant trastuzumab
will hold up over time?
DR LOVE: How do you think the efficacy data for adjuvant trastuzumab
will hold up over time?
![]() DR SLAMON: We don’t yet know if the 50 percent reduction in the event rate
for the patients who received adjuvant trastuzumab will hold up longitudinally.
The other studies will not be able to answer that question because they
allowed patients to cross over to trastuzumab, but BCIRG 006 will provide
that answer because we had very few crossovers.
DR SLAMON: We don’t yet know if the 50 percent reduction in the event rate
for the patients who received adjuvant trastuzumab will hold up longitudinally.
The other studies will not be able to answer that question because they
allowed patients to cross over to trastuzumab, but BCIRG 006 will provide
that answer because we had very few crossovers.
![]() DR LOVE: Why weren’t there many crossovers in the BCIRG trial?
DR LOVE: Why weren’t there many crossovers in the BCIRG trial?
![]() DR SLAMON: When it became evident that the adjuvant studies were positive,
patients in the control groups on HERA and the US trials were offered trastuzumab.
A number of those patients then received trastuzumab.
DR SLAMON: When it became evident that the adjuvant studies were positive,
patients in the control groups on HERA and the US trials were offered trastuzumab.
A number of those patients then received trastuzumab.
On the other hand, our trial accrued quickly, and in less than three years, we had enrolled all 3,222 patients. We were waiting for our data to mature when the two US groups combined theirs. Our patients were far out from their adjuvant chemotherapy, and only a handful of patients in the BCIRG 006 trial crossed over. So this 3,000+ patient study will tell us how those hazard ratios stand up over time.
Tracks 8, 10
![]() DR LOVE: How do you feel about using trastuzumab in patients with
lower-risk, node-negative disease?
DR LOVE: How do you feel about using trastuzumab in patients with
lower-risk, node-negative disease?
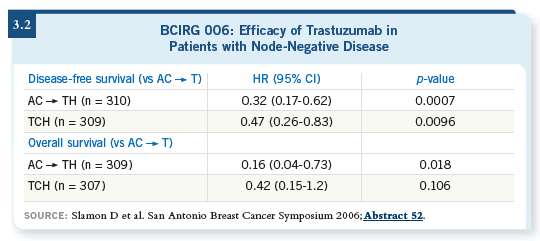
![]() DR SLAMON: The HERA and the BCIRG trials were the only two that
enrolled patients with node-negative disease. The HERA trial shows an
equivalent benefit for patients with node-negative and node-positive disease.
The data for the patients with node-negative disease in the BCIRG trial,
which was 30 percent of the patients, will be released at the San Antonio
Breast Cancer Symposium this year (Slamon 2006; [3.2]).
DR SLAMON: The HERA and the BCIRG trials were the only two that
enrolled patients with node-negative disease. The HERA trial shows an
equivalent benefit for patients with node-negative and node-positive disease.
The data for the patients with node-negative disease in the BCIRG trial,
which was 30 percent of the patients, will be released at the San Antonio
Breast Cancer Symposium this year (Slamon 2006; [3.2]).
![]() DR LOVE: Are there any cases of HER2-positive, node-negative breast cancer
in which you would not use adjuvant trastuzumab?
DR LOVE: Are there any cases of HER2-positive, node-negative breast cancer
in which you would not use adjuvant trastuzumab?
![]() DR SLAMON: The only type of case where I would consider not using trastuzumab
— and it’s a judgment call — is DCIS with an area of microinvasion.
If there’s a frank, invasive carcinoma that’s HER2-positive, I don’t care about
the size. That tumor, in my opinion, is a HER2-driven tumor and should be
treated accordingly.
DR SLAMON: The only type of case where I would consider not using trastuzumab
— and it’s a judgment call — is DCIS with an area of microinvasion.
If there’s a frank, invasive carcinoma that’s HER2-positive, I don’t care about
the size. That tumor, in my opinion, is a HER2-driven tumor and should be
treated accordingly.
![]() DR LOVE: In that situation, does it matter to you whether the tumor is
ER-positive or ER-negative?
DR LOVE: In that situation, does it matter to you whether the tumor is
ER-positive or ER-negative?
![]() DR SLAMON: No.
DR SLAMON: No.
Track 11
![]() DR LOVE: From a cardiac perspective, is docetaxel safer than paclitaxel in
a trastuzumab-based regimen?
DR LOVE: From a cardiac perspective, is docetaxel safer than paclitaxel in
a trastuzumab-based regimen?
![]() DR SLAMON: The data from the trastuzumab adjuvant trials and clinical
experience clearly indicate that docetaxel may be less cardiotoxic than
paclitaxel. Both taxanes have similar mechanisms of action but different
toxicity profiles.
DR SLAMON: The data from the trastuzumab adjuvant trials and clinical
experience clearly indicate that docetaxel may be less cardiotoxic than
paclitaxel. Both taxanes have similar mechanisms of action but different
toxicity profiles.
As for the cardiac issue, data indicate that docetaxel may be less cardiotoxic, although much of that comes from cross-trial comparison. The fact that we saw this big difference and that the adjuvant trastuzumab trials were conducted almost simultaneously with similar criteria for monitoring cardiac function in identical groups of women made us start to consider that docetaxel is a less cardiotoxic drug.
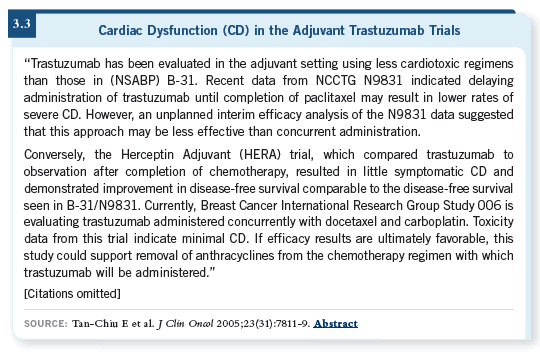
In the Journal of Clinical Oncology publication of the NSABP-B-31 data, the incidence of cardiac dysfunction of any type was 34 percent among the patients who received an anthracycline with trastuzumab (Tan-Chiu 2005; [3.3]). One third of the patients are developing some signal, disproportionate to what you see in the nontrastuzumab-containing arm, where it’s closer to 12 or 13 percent.
The rate of cardiac dysfunction in our study was 17 percent of the patients, so again it was about half. It’s remarkable how these numbers have stayed consistent. In clinical cardiotoxicity, the difference is about twofold. In subclinical cardiotoxicity, it’s also twofold. So this isn’t just noise. I believe it’s a real signal that we’re getting from the clinical data sets.
![]() DR LOVE: Do you think this is relevant only when trastuzumab is on board or
in general?
DR LOVE: Do you think this is relevant only when trastuzumab is on board or
in general?
![]() DR SLAMON: I believe it’s particularly relevant when trastuzumab is on board,
but that tends to make us believe that docetaxel is a less cardiotoxic agent.
DR SLAMON: I believe it’s particularly relevant when trastuzumab is on board,
but that tends to make us believe that docetaxel is a less cardiotoxic agent.
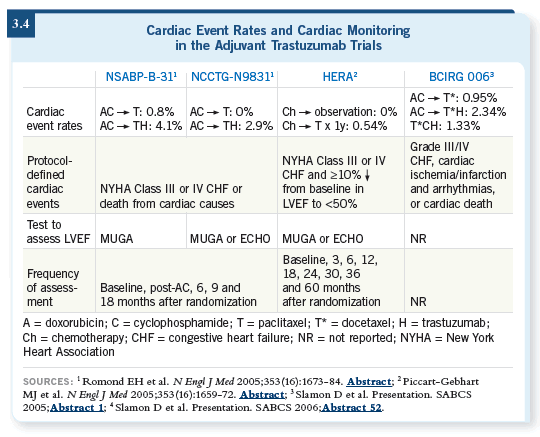
This audio program on cardiac issues is timely because of the important messages that have come out recently, which have been a wake-up call for oncologists (3.4). We came to realize that treatment doesn’t only involve getting patients through their neutropenia and fevers and then they get better.
Patients are incurring some longer-term damage that we need to be aware of, so as we introduce the new, targeted therapies — such as the small-molecule tyrosine kinase inhibitors like sorafenib or sunitinib — we realize we may be potentiating cardiac dysfunction when we begin with these cardiac-injuring drugs.
Track 12
![]() DR LOVE: Trastuzumab monotherapy has been heavily debated in terms
of clinical practice. Will this be evaluated in a clinical trial?
DR LOVE: Trastuzumab monotherapy has been heavily debated in terms
of clinical practice. Will this be evaluated in a clinical trial?
![]() DR SLAMON: It absolutely should be studied. Clinicians use it in practice now
in isolated cases of elderly patients with HER2-positive tumors who might not
tolerate chemotherapy but are otherwise healthy.
DR SLAMON: It absolutely should be studied. Clinicians use it in practice now
in isolated cases of elderly patients with HER2-positive tumors who might not
tolerate chemotherapy but are otherwise healthy.
The risk factors for cardiac dysfunction are hypertension, low baseline LVEF and age. They appear to be almost equal in their contribution, and they are cumulative.
So in patients who have all of those factors, the incidence of cardiac dysfunction is high. For some of the patients who have all of those factors but in particular hypertension and advanced age, the incidence of clinical congestive heart failure goes up to about 10 percent.
Everyone agrees that a one-in-10 risk of cardiac dysfunction in the adjuvant setting is unacceptable. We “dialed up” the efficacy nicely, and I believe we may be reaching the ceiling, and now it’s time to consider “dialing back” on the toxicity.
![]() DR LOVE: Do we have any idea of what we might be losing, in terms of
antitumor effect, by not using chemotherapy?
DR LOVE: Do we have any idea of what we might be losing, in terms of
antitumor effect, by not using chemotherapy?
![]() DR SLAMON: No, I don’t have any idea. We need a clinical trial of adjuvant
trastuzumab monotherapy versus the standard approach. I believe that trial
will be conducted, despite the fact that the regulators and the physicians who
presume to speak for the patients say that patients absolutely will not accept
taking trastuzumab without chemotherapy.
DR SLAMON: No, I don’t have any idea. We need a clinical trial of adjuvant
trastuzumab monotherapy versus the standard approach. I believe that trial
will be conducted, despite the fact that the regulators and the physicians who
presume to speak for the patients say that patients absolutely will not accept
taking trastuzumab without chemotherapy.
When you actually talk with large groups of patients and ask if they would be willing to take this risk and eliminate chemotherapy, the answer comes back resoundingly, “Yes.” We believe that’s a question worth asking and answering.
![]() DR LOVE: Do you think such a trial will be conducted?
DR LOVE: Do you think such a trial will be conducted?
![]() DR SLAMON: I hope so. In the BCIRG, we’re talking about trying to do
something along those lines.
DR SLAMON: I hope so. In the BCIRG, we’re talking about trying to do
something along those lines.