
 |
| Tracks 1-14 | ||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 2-3
![]() DR LOVE: Can you provide an overview of anthracycline-related
cardiotoxicity?
DR LOVE: Can you provide an overview of anthracycline-related
cardiotoxicity?
![]() DR STEINGART: There are acute, subacute and long-term anthracycline-associated
cardiotoxicities. The acute toxicity may be associated with arrhythmias
and chest pain. It looks like an inflammatory response and occurs within
hours or days of the administration of an anthracycline. The subacute toxicity
is described as heart failure at a month or more thereafter.
DR STEINGART: There are acute, subacute and long-term anthracycline-associated
cardiotoxicities. The acute toxicity may be associated with arrhythmias
and chest pain. It looks like an inflammatory response and occurs within
hours or days of the administration of an anthracycline. The subacute toxicity
is described as heart failure at a month or more thereafter.
Long-term toxicity occurs when individuals who have received an anthracycline and are not suspected of having any cardiac reaction end up with heart failure years later. Whether those are separate entities or simply clinical manifestations of the fundamental toxicity showing themselves at different time points in the disease is unclear in my mind.
I conceptualize the events as part of the same entity. It is a matter of how sensitive the patient is to the drug and whether there’s a substrate for it to manifest clinically. I don’t know that there’s any fundamentally different pathophysiologic basis behind those three arbitrarily defined stages.
![]() DR LOVE: What do we know about its molecular pathogenesis?
DR LOVE: What do we know about its molecular pathogenesis?
![]() DR STEINGART: It seems to be related to the requirement for elemental iron
and dangerous free-radical oxygen species that cause fundamental mitochondrial
damage to the myocyte. That is the premise behind dexrazoxane, which
has been advocated as a means of ameliorating the cardiotoxicity. If you
remove iron from the environment where anthracyclines are working, you can
alleviate some of the cardiotoxicity through production of fewer free radicals,
less damage to the mitochondria, less myocardial cell death and less fibrosis.
DR STEINGART: It seems to be related to the requirement for elemental iron
and dangerous free-radical oxygen species that cause fundamental mitochondrial
damage to the myocyte. That is the premise behind dexrazoxane, which
has been advocated as a means of ameliorating the cardiotoxicity. If you
remove iron from the environment where anthracyclines are working, you can
alleviate some of the cardiotoxicity through production of fewer free radicals,
less damage to the mitochondria, less myocardial cell death and less fibrosis.
Track 4
![]() DR LOVE: Can you summarize the studies evaluating anthracycline-related
cardiotoxicity that were presented at ASCO 2006?
DR LOVE: Can you summarize the studies evaluating anthracycline-related
cardiotoxicity that were presented at ASCO 2006?
![]() DR STEINGART: Dr Giordano from MD Anderson used the SEER-Medicare
database to consider women older than 65 years of age who had received an
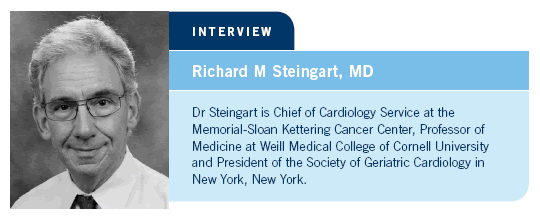
adjuvant anthracycline and were followed long term. It is exciting to have
five- and 10-year data for women with breast cancer (Giordano 2006; [1.1]).
DR STEINGART: Dr Giordano from MD Anderson used the SEER-Medicare
database to consider women older than 65 years of age who had received an
adjuvant anthracycline and were followed long term. It is exciting to have
five- and 10-year data for women with breast cancer (Giordano 2006; [1.1]).
The goal of the study was to determine whether anthracyclines were associated with long-term congestive heart failure in two distinct age groups — 66 to 70 years old and over 70 years old. They divided the elderly into those age groups because they found an interaction between age and chemotherapy (Giordano 2006).
For women aged 66 to 70 years, those receiving an adjuvant anthracycline compared to those receiving an adjuvant nonanthracycline or no adjuvant chemotherapy had a higher rate of clinical congestive heart failure. At 10 years, more than 40 percent of the women between 66 and 70 years of age who received an adjuvant anthracycline had a clinical diagnosis of congestive heart failure (Giordano 2006; [1.1]).
Patients older than 70 years of age also had a very high incidence of congestive heart failure, but it didn’t matter whether they received an anthracycline or a nonanthracycline-containing regimen or no chemotherapy (Giordano 2006).
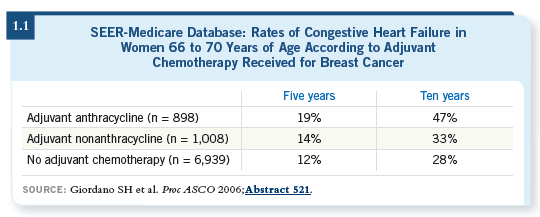
A remarkably high incidence of clinical congestive heart failure was diagnosed in the Medicare database. Cofactors associated with congestive heart failure included hypertension, diabetes and coronary artery disease (Giordano 2006; [1.2]).
![]() DR LOVE: It was counterintuitive
that the difference
wasn’t seen in the women
older than 70 years of age.
Do you think this might
have been related to the
background incidence of CHF
or maybe selection bias?
DR LOVE: It was counterintuitive
that the difference
wasn’t seen in the women
older than 70 years of age.
Do you think this might
have been related to the
background incidence of CHF
or maybe selection bias?
![]() DR STEINGART: The authors
speculated it was the result of
selection bias. Patients older
than 70 years of age who
received an anthracycline
were so carefully screened
that they were materially
different from the patients
who did not receive an
anthracycline — they were
healthier.
DR STEINGART: The authors
speculated it was the result of
selection bias. Patients older
than 70 years of age who
received an anthracycline
were so carefully screened
that they were materially
different from the patients
who did not receive an
anthracycline — they were
healthier.
Whatever increase in the incidence of cardiomyopathy there may have been from the anthracycline in that group was offset by other risk factors in the patients who did not receive anthracyclines (Giordano 2006).
Tracks 5-6
![]() DR LOVE: Can you discuss the correlation between a decline in ejection
fraction and congestive heart failure?
DR LOVE: Can you discuss the correlation between a decline in ejection
fraction and congestive heart failure?
![]() DR STEINGART: We spend a great deal of time monitoring the ejection
fraction when we administer chemotherapeutic agents. Declines in ejection fraction with anthracyclines are associated with adverse outcomes, mortality
and the development of clinical congestive heart failure.
DR STEINGART: We spend a great deal of time monitoring the ejection
fraction when we administer chemotherapeutic agents. Declines in ejection fraction with anthracyclines are associated with adverse outcomes, mortality
and the development of clinical congestive heart failure.
When you start getting into the older age groups, however, declines in ejection fraction and heart failure are different concepts. Most of the heart failure in women older than 65 years of age occurs in the presence of a normal ejection fraction.
![]() DR LOVE: Is that specific to women?
DR LOVE: Is that specific to women?
![]() DR STEINGART: It’s specific to women in the sense that most of the heart
failure in women older than 65 years of age is the consequence of hypertension.
In men, it’s a consequence of a mixture of hypertension and coronary
artery disease.
DR STEINGART: It’s specific to women in the sense that most of the heart
failure in women older than 65 years of age is the consequence of hypertension.
In men, it’s a consequence of a mixture of hypertension and coronary
artery disease.
Older men are more likely than younger men to develop heart failure with a normal ejection fraction. However, there is still a slight preponderance of heart failure with a depressed ejection fraction because coronary artery disease is still the driving force in heart failure in older men.
In women, however, the driving force for congestive heart failure is far and away hypertension. Those women present with concentric left ventricular hypertrophy, normal-sized cardiac cavities, thick walls, inability to fill the ventricle properly and backward heart failure, pulmonary congestion, edema and exercise intolerance.
If one carefully examines the literature, one sees that anthracyclines not only affect the ejection fraction, but they also affect the diastolic properties of the left ventricle.
As women age, they may develop hypertension, coronary disease or diabetes. The coalescence of those factors with anthracycline toxicity can produce the clinical entity of congestive heart failure.
That is an important diagnosis because recent data from the Mayo Clinic and Canada, published in The New England Journal of Medicine, show that the prognosis of congestive heart failure with a normal ejection fraction is the same as that of congestive heart failure with a depressed ejection fraction (Bhatia 2006; Owan 2006).
Track 7
![]() DR LOVE: What are the chances that a 55-year-old woman without any
comorbidities will develop heart failure over the next 20 years as a result
of four cycles of AC?
DR LOVE: What are the chances that a 55-year-old woman without any
comorbidities will develop heart failure over the next 20 years as a result
of four cycles of AC?
![]() DR STEINGART: I can’t answer that question. The data I described from the
SEER-Medicare database were with women who started adjuvant treatment
at 65 years of age or older (Giordano 2006). What happens to the 55-year-old
who receives treatment and lives to 65 or 75 years of age is unclear. I don’t
believe it’s unreasonable, however, to extrapolate from the data with women
older than 65 years of age.
DR STEINGART: I can’t answer that question. The data I described from the
SEER-Medicare database were with women who started adjuvant treatment
at 65 years of age or older (Giordano 2006). What happens to the 55-year-old
who receives treatment and lives to 65 or 75 years of age is unclear. I don’t
believe it’s unreasonable, however, to extrapolate from the data with women
older than 65 years of age.
![]() DR LOVE: Based on Patterns of Care surveys we have conducted, the most
common answer an oncologist in this country will provide to this question is
one or two percent. Do you think that is correct?
DR LOVE: Based on Patterns of Care surveys we have conducted, the most
common answer an oncologist in this country will provide to this question is
one or two percent. Do you think that is correct?
![]() DR STEINGART: The excess risk due to doxorubicin — at 240 mg/m2 — is
somewhere between one and four percent, in the shorter term, over three to
five years. However, if you obtain a biopsy from these women and do careful
measurements of left ventricular function, you will find abnormalities of
cardiac structure and function in the majority of them.
DR STEINGART: The excess risk due to doxorubicin — at 240 mg/m2 — is
somewhere between one and four percent, in the shorter term, over three to
five years. However, if you obtain a biopsy from these women and do careful
measurements of left ventricular function, you will find abnormalities of
cardiac structure and function in the majority of them.
Then you add to that the rising incidence of hypertension over the next five to 20 years, which goes as high as 75 to 85 percent of women by the time they reach the age of 85. The probability of excess risk of developing congestive heart failure is dramatically higher than simply one or two percent.
![]() DR LOVE: What about in a 65-year-old woman without any comorbidities?
DR LOVE: What about in a 65-year-old woman without any comorbidities?
![]() DR STEINGART: For 65-year-old patients, the data are better. If they received
an anthracycline, probably 30 to 40 percent will have a clinical diagnosis of
congestive heart failure over the next 10 to 20 years.
DR STEINGART: For 65-year-old patients, the data are better. If they received
an anthracycline, probably 30 to 40 percent will have a clinical diagnosis of
congestive heart failure over the next 10 to 20 years.
Tracks 10-11
![]() DR LOVE: What are the mechanisms involved with trastuzumab-related
cardiotoxicity?
DR LOVE: What are the mechanisms involved with trastuzumab-related
cardiotoxicity?
![]() DR STEINGART: Trastuzumab appears to work by blocking cell-repair
pathways when it blocks the HER2 receptor. It appears to turn off a mechanism
that is designed to prevent programmed cell death. Trastuzumab is not a
primary cardiotoxin, like an anthracycline. However, if a cell is already in the
process of distress, trastuzumab will accelerate cell death or prevent recovery
of normal cellular function because it blocks reparative pathways.
DR STEINGART: Trastuzumab appears to work by blocking cell-repair
pathways when it blocks the HER2 receptor. It appears to turn off a mechanism
that is designed to prevent programmed cell death. Trastuzumab is not a
primary cardiotoxin, like an anthracycline. However, if a cell is already in the
process of distress, trastuzumab will accelerate cell death or prevent recovery
of normal cellular function because it blocks reparative pathways.
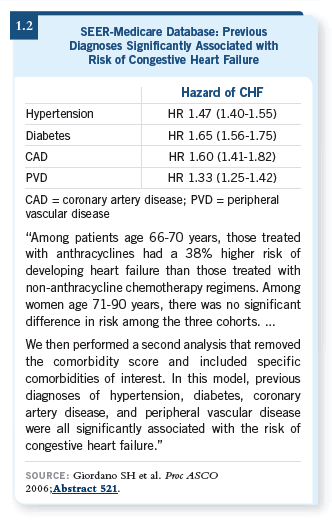
NSABP-B-31 was thoughtfully constructed to assess potential cardiac toxicity. In that trial, when the ejection fraction fell according to prespecified parameters, trastuzumab was stopped and cardiac monitoring was continued. In the vast majority of patients, left ventricular function returned to normal (Tan-Chiu 2005; [1.3]), which supports the hypothesis of cellular repair.
Michael Ewer produced biopsy data for women with depressed ejection fractions who had received an anthracycline and trastuzumab. No obvious changes were evident by light microscopy (Ewer 2005).
More recent data do show abnormalities on electron microscopy with trastuzumab, but those abnormalities have been characterized as what is generally associated with reversible cardiomyopathy (Guarneri 2006).
Those studies include few biopsies, and the data are early. Researchers have not been able to discover a permanent, structural lesion in the cell that has become dysfunctional as a consequence of trastuzumab. The hope is that function will improve on the withdrawal of trastuzumab.
In the combined analysis of NSABP-B-31 and NCCTG-N9831, approximately 18 percent of the women with normal ejection fractions following the anthracycline phase of their treatment discontinued trastuzumab because they either had clinical congestive heart failure or their ejection fraction fell beyond a prespecified parameter (Romond 2005).
![]() DR LOVE: What do we know about the reintroduction of trastuzumab in
patients whose ejection fraction dropped within the normal range?
DR LOVE: What do we know about the reintroduction of trastuzumab in
patients whose ejection fraction dropped within the normal range?
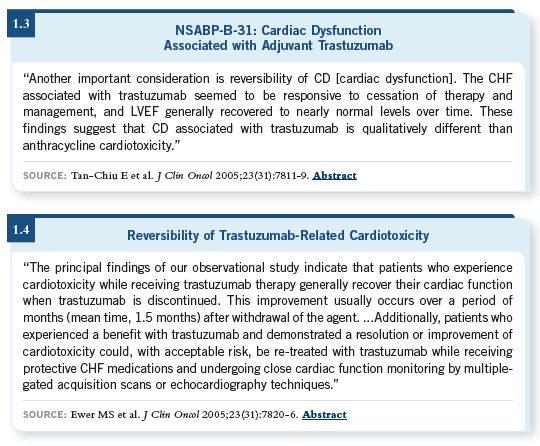
![]() DR STEINGART: We don’t know a lot about the reintroduction of trastuzumab
from the randomized trials. We do know about it from our clinic’s experience
and a publication in the Journal of Clinical Oncology (Ewer 2005; [1.4]). For the
most part, in the metastatic breast cancer setting, where there’s greater experience,
the vast majority of women tolerated the reintroduction of trastuzumab
with or without treatment for left ventricular systolic dysfunction with beta
blockers, ACE inhibitors or angiotensin receptor blockers (Ewer 2005; [1.4]).
DR STEINGART: We don’t know a lot about the reintroduction of trastuzumab
from the randomized trials. We do know about it from our clinic’s experience
and a publication in the Journal of Clinical Oncology (Ewer 2005; [1.4]). For the
most part, in the metastatic breast cancer setting, where there’s greater experience,
the vast majority of women tolerated the reintroduction of trastuzumab
with or without treatment for left ventricular systolic dysfunction with beta
blockers, ACE inhibitors or angiotensin receptor blockers (Ewer 2005; [1.4]).
Track 12
![]() DR LOVE: What about the use of trastuzumab or an anthracycline for
patients with prior cardiac events but normal ejection fractions?
DR LOVE: What about the use of trastuzumab or an anthracycline for
patients with prior cardiac events but normal ejection fractions?
![]() DR STEINGART: In the world of cardiology, there’s very little published
experience with that issue. It is a key question. If there’s no significant valvular disease in a patient who has had a small infarct or some other insult, the tolerability
of the toxicity will be the same as long as the ejection fraction is within
the normal range of 55 percent or higher. I would imagine that trastuzumab
or an anthracycline could be tolerated under those circumstances.
DR STEINGART: In the world of cardiology, there’s very little published
experience with that issue. It is a key question. If there’s no significant valvular disease in a patient who has had a small infarct or some other insult, the tolerability
of the toxicity will be the same as long as the ejection fraction is within
the normal range of 55 percent or higher. I would imagine that trastuzumab
or an anthracycline could be tolerated under those circumstances.
![]() DR LOVE: Is trastuzumab absolutely contraindicated in a patient with HER2-positive, metastatic breast cancer who has cardiomyopathy or cardiac problems?
DR LOVE: Is trastuzumab absolutely contraindicated in a patient with HER2-positive, metastatic breast cancer who has cardiomyopathy or cardiac problems?
![]() DR STEINGART: Absolutely not. It’s a negotiation on a patient-by-patient
basis. We clearly continue to recommend using trastuzumab in the palliative
care setting for patients with ejection fractions in the 40s or compensated
congestive heart failure. One of the challenges of being a cardiologist or an
oncologist is striking the proper balance with those patients.
DR STEINGART: Absolutely not. It’s a negotiation on a patient-by-patient
basis. We clearly continue to recommend using trastuzumab in the palliative
care setting for patients with ejection fractions in the 40s or compensated
congestive heart failure. One of the challenges of being a cardiologist or an
oncologist is striking the proper balance with those patients.
If the patient has bone pain, a pleural effusion or symptomatic abnormalities from the metastatic cancer that may be relieved by trastuzumab, we’re no longer talking about differences in survival under those circumstances. We’re talking about improving the quality of life. Trading the possibility of congestive heart failure for an improvement in the quality of life from the metastatic breast cancer is well worth a discussion between the patient and the oncologist.