
 |

| Tracks 1-31 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Tracks 2-3
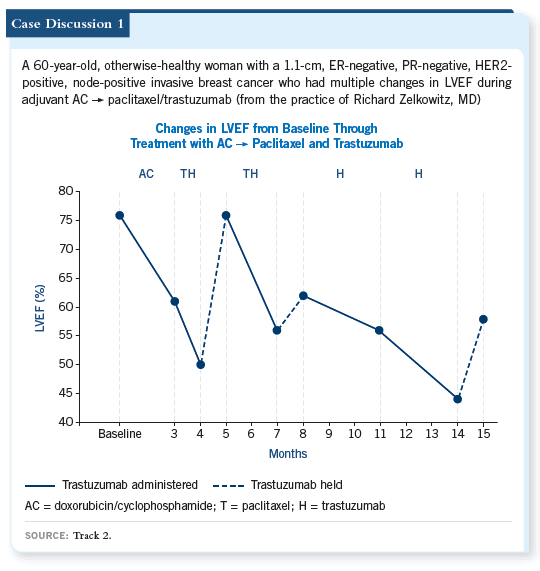
![]() DR LOVE: Hal, how often do you see significant changes in the ejection
fraction in patients treated with trastuzumab?
DR LOVE: Hal, how often do you see significant changes in the ejection
fraction in patients treated with trastuzumab?
![]() DR BURSTEIN: I have found, especially with an LVEF greater than 70 percent,
that there’s a lot of noise and it is a hyperdynamic number. I wonder whether
these drops from 76 to 61 percent are real events. However, dropping below 50
percent seems more real and would be worrisome, especially if it were linked to
symptoms and obvious changes on an echocardiogram.
DR BURSTEIN: I have found, especially with an LVEF greater than 70 percent,
that there’s a lot of noise and it is a hyperdynamic number. I wonder whether
these drops from 76 to 61 percent are real events. However, dropping below 50
percent seems more real and would be worrisome, especially if it were linked to
symptoms and obvious changes on an echocardiogram.
![]() DR LOVE: Dr Steingart, can you talk about the variation in ejection fraction,
particularly within the normal range?
DR LOVE: Dr Steingart, can you talk about the variation in ejection fraction,
particularly within the normal range?
![]() DR STEINGART: We have discovered that when the ejection fraction is 70
percent or higher, there is enormous variability from one measurement to the next. If you measure serial ejection fractions, a 15-unit ejection fraction
change is not unusual.
DR STEINGART: We have discovered that when the ejection fraction is 70
percent or higher, there is enormous variability from one measurement to the next. If you measure serial ejection fractions, a 15-unit ejection fraction
change is not unusual.
At a very high ejection fraction in a normal-sized heart, you’re talking about an end-systolic dimension that is literally a pixel on the computer matrix. Any variation in the detection of the edge around that one pixel will make it grow from one to three or four pixels.
With very small end-systolic volumes in a healthy heart, minor variations in your edge detection ability can produce dramatic changes in the ejection fraction. So, in practice, we are skeptical about these fluctuations within the normal range.
Track 4
![]() DR LOVE: Dennis, what chemo/trastuzumab regimen would you have
used with this patient?
DR LOVE: Dennis, what chemo/trastuzumab regimen would you have
used with this patient?
![]() DR SLAMON: I believe you can go with either of the BCIRG 006 trastuzumab
regimens, and patients should be aware of the differences in efficacy
and toxicity between the regimens.
DR SLAMON: I believe you can go with either of the BCIRG 006 trastuzumab
regimens, and patients should be aware of the differences in efficacy
and toxicity between the regimens.
In addition, although we don’t have data, I believe docetaxel/cyclophosphamide, the regimen Steve Jones evaluated ( Jones SE 2006), would be equally efficacious because the taxane is the same and cyclophosphamide has additive and synergistic interactions with trastuzumab.
![]() DR LOVE: I believe US Oncology is planning to study docetaxel/cyclophosphamide
with trastuzumab. Would you be comfortable using it right now?
DR LOVE: I believe US Oncology is planning to study docetaxel/cyclophosphamide
with trastuzumab. Would you be comfortable using it right now?
![]() DR SLAMON: My sense is that the chemotherapy platform you build trastuzumab
on is much less important than using trastuzumab and using it for an
optimum period of time, which has yet to be determined. For a HER2-driven
tumor, that is the critical factor.
DR SLAMON: My sense is that the chemotherapy platform you build trastuzumab
on is much less important than using trastuzumab and using it for an
optimum period of time, which has yet to be determined. For a HER2-driven
tumor, that is the critical factor.
![]() DR LOVE: Why would you use docetaxel as opposed to paclitaxel?
DR LOVE: Why would you use docetaxel as opposed to paclitaxel?
![]() DR SLAMON: The cardiotoxicity data we’re seeing from all the trials show less
cardiotoxicity in the one trial using docetaxel (Slamon 2005) compared to all
the trials using paclitaxel (Romond 2005; Piccart-Gebhart 2005). Everyone
who’s observed the data from all those different groups is saying the same
thing — the only difference was the taxane.
DR SLAMON: The cardiotoxicity data we’re seeing from all the trials show less
cardiotoxicity in the one trial using docetaxel (Slamon 2005) compared to all
the trials using paclitaxel (Romond 2005; Piccart-Gebhart 2005). Everyone
who’s observed the data from all those different groups is saying the same
thing — the only difference was the taxane.
![]() DR BURSTEIN: My most common trastuzumab-based regimen remains AC
followed by paclitaxel, which is similar to the regimen used in the Inter-group
study (Romond 2005). We participated in that trial and treated a lot of
patients with every three-week AC times four followed by weekly paclitaxel
times 12.
DR BURSTEIN: My most common trastuzumab-based regimen remains AC
followed by paclitaxel, which is similar to the regimen used in the Inter-group
study (Romond 2005). We participated in that trial and treated a lot of
patients with every three-week AC times four followed by weekly paclitaxel
times 12.
![]() DR LOVE: Do you agree with Dennis’s comment that there seems to be less
cardiotoxicity with the docetaxel regimens than with paclitaxel regimens?
DR LOVE: Do you agree with Dennis’s comment that there seems to be less
cardiotoxicity with the docetaxel regimens than with paclitaxel regimens?
![]() DR BURSTEIN: The incidence of symptomatic cardiac events in the
AC
DR BURSTEIN: The incidence of symptomatic cardiac events in the
AC![]() docetaxel/trastuzumab arm of BCIRG 006 was a little more than two
percent (Slamon 2005). In NSABP-B-31, the absolute incidence of congestive
heart failure symptomatology with AC
docetaxel/trastuzumab arm of BCIRG 006 was a little more than two
percent (Slamon 2005). In NSABP-B-31, the absolute incidence of congestive
heart failure symptomatology with AC![]() paclitaxel/trastuzumab was about
four percent compared to 0.8 percent for the chemotherapy regimen alone
(Tan-Chiu 2005).
paclitaxel/trastuzumab was about
four percent compared to 0.8 percent for the chemotherapy regimen alone
(Tan-Chiu 2005).
I don’t know whether that means there is more or less cardiotoxicity. These patient groups were different. For any number of reasons, it is easy to imagine that they had different preexisting criteria. It’s always tricky to compare across trials.
Track 5
![]() DR LOVE: Dr Steingart, what do we know about the cardiac safety of
dose-dense AC paclitaxel/trastuzumab?
DR LOVE: Dr Steingart, what do we know about the cardiac safety of
dose-dense AC paclitaxel/trastuzumab?
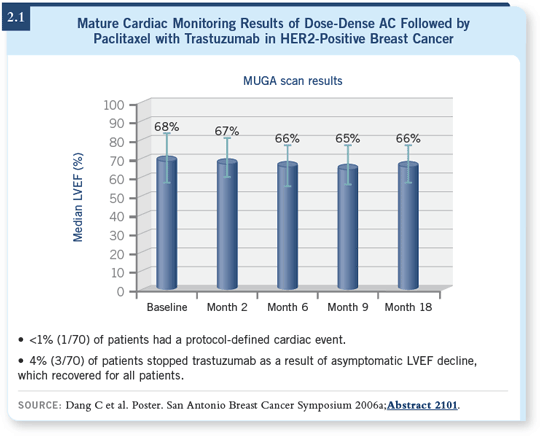
![]() DR STEINGART: We were involved with a recent study of dose-dense
AC
DR STEINGART: We were involved with a recent study of dose-dense
AC![]() paclitaxel/ trastuzumab (Dang 2006a; [2.2]) that utilized stopping
endpoints that were similar to those in the NSABP-B-31 study. The rate of
holding the drug regimen was extremely low, so the dose-dense regimen with
trastuzumab, from a cardiac perspective, in the short term, seemed to be well
tolerated.
paclitaxel/ trastuzumab (Dang 2006a; [2.2]) that utilized stopping
endpoints that were similar to those in the NSABP-B-31 study. The rate of
holding the drug regimen was extremely low, so the dose-dense regimen with
trastuzumab, from a cardiac perspective, in the short term, seemed to be well
tolerated.
![]() DR LOVE: Dennis, is there any theoretical reason to be concerned about closer
spacing of the doxorubicin with the dose-dense regimen?
DR LOVE: Dennis, is there any theoretical reason to be concerned about closer
spacing of the doxorubicin with the dose-dense regimen?
![]() DR SLAMON: I can’t imagine there would be any theoretical reason to be
concerned. It’s clear that the HER2 protein serves a protective function
against stress in the heart. When you have trastuzumab on board, you’re
inhibiting that pathway. You’re inhibiting the pathogenic part of the pathway
with regard to breast cancer, but it appears you’re also inhibiting the positive
effects of the HER2 protein in the heart.
DR SLAMON: I can’t imagine there would be any theoretical reason to be
concerned. It’s clear that the HER2 protein serves a protective function
against stress in the heart. When you have trastuzumab on board, you’re
inhibiting that pathway. You’re inhibiting the pathogenic part of the pathway
with regard to breast cancer, but it appears you’re also inhibiting the positive
effects of the HER2 protein in the heart.
Track 7
![]() DR LOVE: Hal, according to our Patterns of Care studies, in patients
with HER2-negative, node-positive disease, the most common adjuvant
regimen used is dose-dense AC
DR LOVE: Hal, according to our Patterns of Care studies, in patients
with HER2-negative, node-positive disease, the most common adjuvant
regimen used is dose-dense AC![]() paclitaxel. Is that your general approach
for these patients?
paclitaxel. Is that your general approach
for these patients?
![]() DR BURSTEIN: We frequently recommend that regimen for patients with
HER2-negative disease. The Memorial group has reported on dose-dense
AC
DR BURSTEIN: We frequently recommend that regimen for patients with
HER2-negative disease. The Memorial group has reported on dose-dense
AC![]() paclitaxel with trastuzumab in HER2-positive disease, and the data look
encouraging. However, it is a relatively small feasibility study with approximately
70 patients (Dang 2006a; [2.1]). The study is too underpowered to
make strong conclusions about how the safety compares. It’s certainly encouraging
that it looks good.
paclitaxel with trastuzumab in HER2-positive disease, and the data look
encouraging. However, it is a relatively small feasibility study with approximately
70 patients (Dang 2006a; [2.1]). The study is too underpowered to
make strong conclusions about how the safety compares. It’s certainly encouraging
that it looks good.
It’s not clear that dose-dense AC![]() paclitaxel is necessarily better than the
other ways AC
paclitaxel is necessarily better than the
other ways AC![]() paclitaxel was administered in the larger trials for HER 2-positive disease. The only thing that still gives me pause is that you limit your
concurrent chemotherapy/trastuzumab exposure to eight weeks with the dose-dense regimen.
paclitaxel was administered in the larger trials for HER 2-positive disease. The only thing that still gives me pause is that you limit your
concurrent chemotherapy/trastuzumab exposure to eight weeks with the dose-dense regimen.
However, we don’t know if that would be any different from the 12 weeks used in NSABP-B-31, NCCTG -N9831 or BCIRG 006 ( Romond 2005; Slamon 2005). This is a theoretical concern, which isn’t totally trivial because the duration of concurrent therapy might be important.
We need to be candid with patients and say, “These limited data support the regimen in terms of its feasibility.” It is a shorter course of chemotherapy, which is appealing to many patients. It’s not something that we routinely offer outside of a clinical trial.
Track 8
![]() DR LOVE: Dr Steingart, oncologists are trying to follow the adjuvant
trastuzumab trial guidelines in terms of monitoring ejection fractions. Are
there times when the trial guidelines can be stretched?
DR LOVE: Dr Steingart, oncologists are trying to follow the adjuvant
trastuzumab trial guidelines in terms of monitoring ejection fractions. Are
there times when the trial guidelines can be stretched?
![]() DR STEINGART: I don’t fully understand the rationale for the guidelines,
particularly with the change in ejection fraction with in the normal range.
DR STEINGART: I don’t fully understand the rationale for the guidelines,
particularly with the change in ejection fraction with in the normal range.
I don’t understand why one would think a 78 percent ejection fraction that drops to 63 or 62 percent would be a physiologically important change.
I can understand when you start fluctuating near the lower limit of normal, where the room for error is less. If I were consulting on a patient in clinical practice and her ejection fraction went from 78 percent to 62 or 63 percent, assuming there weren’t other pathological indicators on the study, I would not advise the oncologist to stop therapy.
![]() DR LOVE: Dennis, do you agree?
DR LOVE: Dennis, do you agree?
![]() DR SLAMON: I agree that we don’t know what those drops mean. I disagree
when some of that information is interpreted to say that there’s no problem.
DR SLAMON: I agree that we don’t know what those drops mean. I disagree
when some of that information is interpreted to say that there’s no problem.
A definite signal is coming out of the trials that a loss of left ventricular function occurs when these two drugs are used together. It is either clinical or subclinical, but there’s a significant loss of left ventricular function.
We learned this from the 13,000 women in the adjuvant trastuzumab trials, whose median age was 49 years old. The median age of patients with breast cancer is 62 to 63 years. So the trials included younger women, and we’re seeing a signal. They have not yet accrued their other life events that could affect cardiac function.
If this is a real signal and it’s sustained, it may be a problem. If it’s not and they all recover, then “no harm, no foul.” The efficacy data are incredible, but the efficacy has to be weighed against the toxicity. Trastuzumab has no bigger advocate than me, but I’ve tried to be cautious about saying that there’s no issue when there are subclinical losses above the lower limits of normal.
![]() DR LOVE: When you make that decision, how do you factor in the risk of
recurrence — for example, a patient with 10 versus zero positive nodes?
DR LOVE: When you make that decision, how do you factor in the risk of
recurrence — for example, a patient with 10 versus zero positive nodes?
![]() DR SLAMON: A HER2-positive tumor is a HER2-positive tumor. To me
— and I may be in the minority — the number of nodes is irrelevant. The data
demonstrate that node-negative disease that is HER2-positive behaves as if it is
node-positive disease, even if the tumor is smaller than 1.5 centimeters.
DR SLAMON: A HER2-positive tumor is a HER2-positive tumor. To me
— and I may be in the minority — the number of nodes is irrelevant. The data
demonstrate that node-negative disease that is HER2-positive behaves as if it is
node-positive disease, even if the tumor is smaller than 1.5 centimeters.
When I see a patient with a HER2-positive tumor, even when it is 0.5 centimeters, the argument I make is, “This is a HER2-driven tumor. We would recommend trastuzumab-based therapy.”
Track 9
![]() DR LOVE: Hal, people have pointed out what they thought was a blip up
in the recurrence rate in the HERA study after trastuzumab was stopped
at a year. Do you think that’s real?
DR LOVE: Hal, people have pointed out what they thought was a blip up
in the recurrence rate in the HERA study after trastuzumab was stopped
at a year. Do you think that’s real?
![]() DR BURSTEIN: I don’t know. The worry has been that chronic suppression
is necessary and if you withdraw trastuzumab, you might put the patient in
greater jeopardy for recurrence.
DR BURSTEIN: I don’t know. The worry has been that chronic suppression
is necessary and if you withdraw trastuzumab, you might put the patient in
greater jeopardy for recurrence.
As a practical matter, the trials had to start somewhere. Most of the large cooperative group trials administered one year of therapy. The HERA trial had a randomization to one versus two years (Piccart-Gebhart 2005; Smith 2007).
A challenge in interpreting that literature, when it finally matures, is that they did not administer chemotherapy concurrently with trastuzumab in the HERA trial. I remain concerned that that may be a less optimal way to utilize trastuzumab.
Although there may be some advantage for a second year, it will not be clear that the advantage would be the same if you used chemotherapy and trastuzumab concurrently. This is something that will have to be teased out once the more mature data become available. For the moment, outside of a clinical trial, I use one year of trastuzumab.
![]() DR SLAMON: I believe Hal’s point is well made; those data definitely need
to be teased out. There’s one other big problem with the HERA trial. It’s a
wonderful trial showing that if you use trastuzumab after chemotherapy, you
will still have a clinical benefit.
DR SLAMON: I believe Hal’s point is well made; those data definitely need
to be teased out. There’s one other big problem with the HERA trial. It’s a
wonderful trial showing that if you use trastuzumab after chemotherapy, you
will still have a clinical benefit.
What is factored out of the HERA trial, however, which few people mention, is that the randomization occurred after patients finished their surgery, chemotherapy and radiation. That could have taken close to 10 months (Piccart-Gebhart 2005; Smith 2007).
In the US cooperative group trials and the BCIRG trial, a number of recurrences occurred early in the patients with HER2-positive disease. In the HERA trial, those patients never went onto the trial. So it’s a favorably selected group in the sense that the worst cases were factored out.
The hazard ratio in HERA is 0.54 (Piccart-Gebhart 2005; Smith 2007), which is a little worse than the hazard ratio we’re seeing in the concurrent trials of around 0.47 to 0.49 (Slamon 2005; Romond 2005). Whether that’s real when comparing across trials is another question.
Track 10
![]() DR LOVE: Hal, should oncologists be following the adjuvant trastuzumab
protocols, in terms of when to monitor ejection fractions and when to stop
and start trastuzumab?
DR LOVE: Hal, should oncologists be following the adjuvant trastuzumab
protocols, in terms of when to monitor ejection fractions and when to stop
and start trastuzumab?
![]() DR BURSTEIN: It’s a tough situation because there is a black-box warning
about cardiotoxicity with the use of trastuzumab. Clearly, these patients merit
cardiac surveillance. It seems as if borderline ejection fraction at baseline, age
and perhaps preexisting hypertension stand out as predictors of trastuzumab-related
cardiomyopathy (Tan-Chiu 2005).
DR BURSTEIN: It’s a tough situation because there is a black-box warning
about cardiotoxicity with the use of trastuzumab. Clearly, these patients merit
cardiac surveillance. It seems as if borderline ejection fraction at baseline, age
and perhaps preexisting hypertension stand out as predictors of trastuzumab-related
cardiomyopathy (Tan-Chiu 2005).
The patients still require surveillance, irrespective of those risk factors. My practicing algorithm is to check cardiac function at baseline, after the anthracycline-based chemotherapy, after three to four months of the taxane/trastuzumab combination and at some point again. It must be said that these safeguards were put in place when we did not know the clinical efficacy of trastuzumab.
The challenge arises in cases with a high-risk breast tumor in which you are trying to bring important therapy to bear on the patient’s disease. When you are trying to combat these reductions in ejection fraction of unknown clinical significance, it’s tough to be a clinician because there aren’t hard and fast rules. The rules in the trials were based on not knowing that trastuzumab was going to be a lifesaving drug for women.
Tracks 12-13
![]() DR LOVE: Dr Steingart, can you talk about idiopathic cardiomyopathy?
What’s the long-term prognosis, and what do we know about the interaction
between it and cardiotoxic agents, such as anthracyclines?
DR LOVE: Dr Steingart, can you talk about idiopathic cardiomyopathy?
What’s the long-term prognosis, and what do we know about the interaction
between it and cardiotoxic agents, such as anthracyclines?
![]() DR STEINGART: We don’t know much about idiopathic dilated cardiomyopathy.
The therapy involves attempting to exclude known causes, such as
alcohol use, hypothyroidism, hyperthyroidism, tachycardia or coronary artery
disease. It’s a diagnosis of exclusion.
DR STEINGART: We don’t know much about idiopathic dilated cardiomyopathy.
The therapy involves attempting to exclude known causes, such as
alcohol use, hypothyroidism, hyperthyroidism, tachycardia or coronary artery
disease. It’s a diagnosis of exclusion.
Because this patient became clinical Class I without further symptoms, her outlook is favorable and is related to the ejection fraction. However, the overall survival of such an individual is not normal.
If one were to biopsy such a heart postmortem, there would be a great deal of fibrosis. The ventricle would be abnormal, and I have concerns about adding a cardiotoxic agent.
I would have a long discussion with the oncologist about the importance of the drug. I believe with this degree of compensation, it wouldn’t take much to further worsen the left ventricular function and make this patient symptomatic again.
This could further accelerate the progression to clinical congestive heart failure, which has an adverse prognosis. I would have substantial reservations about using cardiotoxic chemotherapy in such a patient.
![]() DR LOVE: Hal, how would you think through this case?
DR LOVE: Hal, how would you think through this case?
![]() DR BURSTEIN: Even without the history of the cardiomyopathy, you could
easily convince yourself to just use hormone therapy for this patient. It’s a
low-grade tumor, she’s postmenopausal and her general prognosis is good. The
marginal benefits of adjuvant chemotherapy in a postmenopausal woman with
hormone receptor-positive breast cancer are modest.
DR BURSTEIN: Even without the history of the cardiomyopathy, you could
easily convince yourself to just use hormone therapy for this patient. It’s a
low-grade tumor, she’s postmenopausal and her general prognosis is good. The
marginal benefits of adjuvant chemotherapy in a postmenopausal woman with
hormone receptor-positive breast cancer are modest.
With Peter Ravdin’s Adjuvant! Online, you could estimate the benefit of chemotherapy to be an improvement of a few percentage points in the 10-year disease-free survival. If you really wanted to refine the estimate, you could obtain an Oncotype DX™ assay to quantify the risk.
Given the cardiomyopathy history and what we heard from Dr Steingart about the frailty of these patients, this woman is much more likely to die of her heart disease in the next decade than from her breast cancer, although one would have to look at formal models.
I would be loath to use chemotherapy in this patient, even nonanthracycline-based regimens. Don’t rock this boat. This patient is barely compensated on four cardiac drugs, and you should quit while you’re ahead with the hormone treatment.
Track 14
![]() DR LOVE: Dennis, what are your thoughts about nonanthracycline-containing
alternatives? What about the docetaxel/cyclophosphamide
(TC) regimen Steve Jones presented at the 2005 San Antonio Breast
Cancer Symposium?
DR LOVE: Dennis, what are your thoughts about nonanthracycline-containing
alternatives? What about the docetaxel/cyclophosphamide
(TC) regimen Steve Jones presented at the 2005 San Antonio Breast
Cancer Symposium?
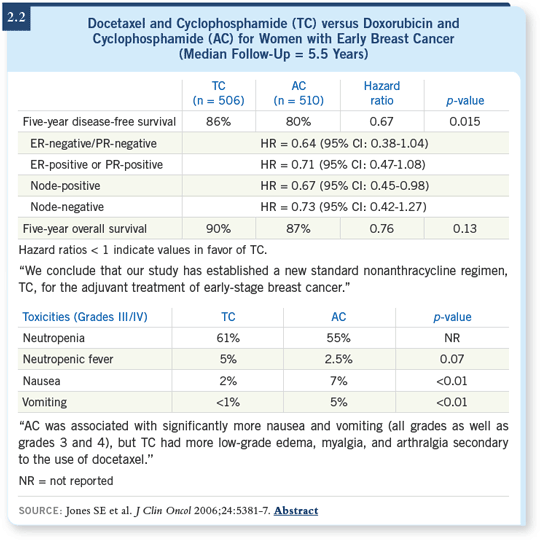
![]() DR SLAMON: Steve Jones’s US Oncology data, comparing TC to anthracycline-based therapy, are impressive ( Jones SE 2006; [2.2]). It’s a large study,
and the data are robust. I believe US Oncology will conduct an even larger
repeat study. I find it a very attractive regimen.
DR SLAMON: Steve Jones’s US Oncology data, comparing TC to anthracycline-based therapy, are impressive ( Jones SE 2006; [2.2]). It’s a large study,
and the data are robust. I believe US Oncology will conduct an even larger
repeat study. I find it a very attractive regimen.
In the case that was just presented, I agree with Hal. An argument would arise as to whether the patient should receive any chemotherapy given the nature of her tumor, the biologic markers and the fact that it’s low-grade, postmenopausal disease. If I were inclined to use chemotherapy, I would not want to administer anything that threatened any minimal cardiac insult.
![]() DR LOVE: Can you follow up on what happened with the patient?
DR LOVE: Can you follow up on what happened with the patient?
![]() DR GEARHART: Although she had low-grade and fairly small disease, we also
recognized that she had at least whatever lymph node burden can be interpreted
from the micrometastasis. We elected to treat her with a nonanthracycline-containing regimen, and we chose docetaxel over paclitaxel, thinking
perhaps we’d slice off another smidgen of cardiac toxicity. She was treated
with Steve Jones’s TC regimen followed by hormonal therapy.
DR GEARHART: Although she had low-grade and fairly small disease, we also
recognized that she had at least whatever lymph node burden can be interpreted
from the micrometastasis. We elected to treat her with a nonanthracycline-containing regimen, and we chose docetaxel over paclitaxel, thinking
perhaps we’d slice off another smidgen of cardiac toxicity. She was treated
with Steve Jones’s TC regimen followed by hormonal therapy.
She actually flew through her TC. We monitored her ejection fraction throughout. She’s now about one year postchemotherapy and continues to have an ejection fraction of about 45 percent and is asymptomatic.
![]() DR LOVE: Dennis, can you comment on the issue of anthracycline
cardiotoxicity in the absence of trastuzumab?
DR LOVE: Dennis, can you comment on the issue of anthracycline
cardiotoxicity in the absence of trastuzumab?
![]() DR SLAMON: The issue of cardiac dysfunction with anthracycline-based
regimens — until the HER2 era — was either enormously underappreciated
or now we’re overestimating it. It’s one of those two extremes, but I’m not
sure which.
DR SLAMON: The issue of cardiac dysfunction with anthracycline-based
regimens — until the HER2 era — was either enormously underappreciated
or now we’re overestimating it. It’s one of those two extremes, but I’m not
sure which.
I was taught in medical school not to worry until you reached more than 450 mg/m2 of doxorubicin. With the regimens consisting of six cycles at 60 mg/m2, we’re at a cumulative dose of 360 mg/m2. When we used four cycles of AC followed by four cycles of T, we were at a cumulative dose of 240 mg/m2. No one ever thought to consider cardiotoxicity.
When we conducted the registrational trial for trastuzumab, we weren’t measuring cardiac dysfunction. No signal came out of the Phase I or Phase II trials. It was during the Phase III trial that women started to say, “Things are going great, except I can’t walk upstairs and I become short of breath, which has never happened to me before.”
We started to look, and we saw this signal. We then planned the adjuvant trastuzumab trials, where everything was followed closely. When we started to follow closely, we found women developing left ventricular dysfunction, some of it significant, at 150 to 180 mg/m2 of doxorubicin.
This is a cardiotoxic drug beyond our traditional thinking. Doxorubicin does bad things to the heart that are independent of the other long-term effects.
Tracks 15-16
![]() DR LOVE: Hal, you have a 60- to 65-year-old patient for whom you’re
recommending either AC alone or dose-dense AC
DR LOVE: Hal, you have a 60- to 65-year-old patient for whom you’re
recommending either AC alone or dose-dense AC![]() paclitaxel. She will
receive four doses of doxorubicin at 60 mg/m2, and she asks, “What are
the chances over the next 20 years, by taking this as opposed to a nonanthracycline
regimen, that I’m going to run into a problem with my heart?”
paclitaxel. She will
receive four doses of doxorubicin at 60 mg/m2, and she asks, “What are
the chances over the next 20 years, by taking this as opposed to a nonanthracycline
regimen, that I’m going to run into a problem with my heart?”
![]() DR BURSTEIN: I usually tell patients that less than one percent will have
clinically significant heart failure through at least a decade of follow-up. As
we’re learning from the SEER-Medicare data set (Giordano 2006; [1.2]) and
the long-term follow-up of some of the original Milan adjuvant trials, there
is a much larger fraction of women who may have late disease or subclinical
changes in LVEF.
DR BURSTEIN: I usually tell patients that less than one percent will have
clinically significant heart failure through at least a decade of follow-up. As
we’re learning from the SEER-Medicare data set (Giordano 2006; [1.2]) and
the long-term follow-up of some of the original Milan adjuvant trials, there
is a much larger fraction of women who may have late disease or subclinical
changes in LVEF.
In the overview analysis, survival at any given time was usually governed by whether the breast cancer recurred. The incidence of excessive deaths from cardiac causes in chemotherapy-treated patients was surprisingly low (EBCTCG 2005). We have a conundrum: Many asymptomatic changes occur, and as women live longer, they seem to be in jeopardy for more late heart failure.
Anthracyclines are undergoing a fascinating revision in the breast cancer literature. Everybody who published articles 15 years ago saying anthracyclines were better than nonanthracycline-based chemotherapy is now publishing papers saying that not everybody needs anthracyclines. We oversold it in the first place.
I believe we will see less use of the anthracyclines, not because of concerns over cardiotoxicity — which are real but fortunately rare — but because we will discover that most women don’t need anthracycline-based chemotherapy.
If you consider many of the major trials that evaluated various doses of anthracyclines, such as MA5 (Levine 2005) and CALGB-8541 (Budman 1998), in the aggregate, anthracyclines clearly helped and that is supported by the overview (EBCTCG 2005).
In many of the retrospective analyses, anthracyclines were most critical in patients with HER2-positive breast cancer (Pritchard 2006). In patients with HER2-negative breast cancer, it’s been hard, for a decade, to see that you actually needed anthracycline-based chemotherapy compared to nonanthracycline-based chemotherapy.
Because those were unplanned, retrospective analyses, those data were not readily accepted. Now we’re coming full circle and there’s more questioning about whether these patients need chemotherapy and if they do, whether they really need anthracycline-based chemotherapy.
Paradoxically, the women who need anthracycline-based chemotherapy are those with HER2-positive disease. We’re going to need the mature data from BCIRG 006 to tease out, within that subgroup, who needs the anthracycline in combination with trastuzumab. For the moment, anthracyclines are the mainstay for HER2-positive disease. I believe we should, as part of this reassessment, be thinking through which breast tumors need anthracycline chemotherapy. It isn’t clear to me that most women do.
![]() DR LOVE: What is your usual regimen for patients with small, HER2-negative, node-negative disease?
DR LOVE: What is your usual regimen for patients with small, HER2-negative, node-negative disease?
![]() DR BURSTEIN: I usually use AC if they’re going to receive chemotherapy.
DR BURSTEIN: I usually use AC if they’re going to receive chemotherapy.
![]() DR LOVE: What were your thoughts about the data, which showed fewer
recurrences with adjuvant TC?
DR LOVE: What were your thoughts about the data, which showed fewer
recurrences with adjuvant TC?
![]() DR BURSTEIN: It’s a provocative study, and I congratulate Steve Jones and the
US Oncology Group for that trial ( Jones SE 2006; [2.2]). We have so many
studies with AC as the backbone — it’s the whole corpus of the NSABP work.
DR BURSTEIN: It’s a provocative study, and I congratulate Steve Jones and the
US Oncology Group for that trial ( Jones SE 2006; [2.2]). We have so many
studies with AC as the backbone — it’s the whole corpus of the NSABP work.
This doesn’t mean that everybody needs it, but it is the backbone of our historical experience in the past 15 or 20 years of chemotherapy. Before giving it up for a well-done but ultimately medium-sized randomized trial, it’s worth looking for confirmatory data. If you want a nonanthracycline-based regimen, you can always use CMF.
![]() DR SLAMON: I disagree with Hal that anthracyclines are undergoing a significant
revision. At some of the centers like his that is the case, but the vast
majority of people feel that if a patient has breast cancer, you “cannot not” use
anthracycline-based therapy. And I disagree with that.
DR SLAMON: I disagree with Hal that anthracyclines are undergoing a significant
revision. At some of the centers like his that is the case, but the vast
majority of people feel that if a patient has breast cancer, you “cannot not” use
anthracycline-based therapy. And I disagree with that.
The data from US Oncology and Steve Jones are compelling. I agree they need to be reproduced, but I suspect that will happen. We’ve been using a drug that has a lot of problems because we were used to using it. These one-size-fits-all approaches for the disease are just wrong.
Track 17
![]() DR LOVE: Dennis, what do you say to a woman who’s about to receive
four cycles of AC in terms of her long-term excess risk of cardiac toxicity?
Is it one percent or 40 percent?
DR LOVE: Dennis, what do you say to a woman who’s about to receive
four cycles of AC in terms of her long-term excess risk of cardiac toxicity?
Is it one percent or 40 percent?
![]() DR SLAMON: I don’t believe for a moment it’s one percent. We’re using old
data sets to make current calls, and women are living into their eighth and
ninth decades. The presentation at ASCO was remarkable and long overdue
(Giordano 2006). It was a high-water mark in terms of our understanding of
the downside effects of what we’re doing.
DR SLAMON: I don’t believe for a moment it’s one percent. We’re using old
data sets to make current calls, and women are living into their eighth and
ninth decades. The presentation at ASCO was remarkable and long overdue
(Giordano 2006). It was a high-water mark in terms of our understanding of
the downside effects of what we’re doing.
I don’t believe the risk is one percent. It’s much higher. The possibility of study bias and the fact that these women were followed closely are real, but usually study bias with such large numbers as were examined in the SEER-Medicare database (Giordano 2006; [1.1]) does not result in such a dramatic difference. So I’d have to believe that the signal is, at least in part, real. It’s not just because the women were followed more closely.
Tracks 19-20
![]() DR LOVE: Dr Steingart, what is the relationship between aortic stenosis
and mantle radiation?
DR LOVE: Dr Steingart, what is the relationship between aortic stenosis
and mantle radiation?
![]() DR STEINGART: A nice series of studies from Stanford evaluated the long-term
effects associated with mantle radiation in patients with Hodgkin’s
disease (Heidenreich 2003). Aortic regurgitation is usually the lesion, but
mixed aortic stenosis and regurgitation is possible.
DR STEINGART: A nice series of studies from Stanford evaluated the long-term
effects associated with mantle radiation in patients with Hodgkin’s
disease (Heidenreich 2003). Aortic regurgitation is usually the lesion, but
mixed aortic stenosis and regurgitation is possible.
She is in the age group where it’s conceivable that she has a congenital bicuspid valve having nothing to do with the therapy. But I believe there’s enough evidence to say that mantle radiation therapy does predispose to aortic valve disease.
![]() DR LOVE: What about the use of an anthracycline or trastuzumab?
DR LOVE: What about the use of an anthracycline or trastuzumab?
![]() DR STEINGART: The ejection fraction is 65 to 75 percent, her dimensions are
normal and she’s not symptomatic. I might put her on a treadmill and gauge
her physiologic response to exercise to convince myself that she had a normal
exercise tolerance. If she did, I would — with one hand on the brake — say it
would be reasonable to administer chemotherapy. I would be concerned that
she would be predisposed to heart failure.
DR STEINGART: The ejection fraction is 65 to 75 percent, her dimensions are
normal and she’s not symptomatic. I might put her on a treadmill and gauge
her physiologic response to exercise to convince myself that she had a normal
exercise tolerance. If she did, I would — with one hand on the brake — say it
would be reasonable to administer chemotherapy. I would be concerned that
she would be predisposed to heart failure.
![]() DR BURSTEIN: She has HER2-overexpressing disease. All of us feel that
trastuzumab is an important treatment for women in this situation. If the
cardiologist clears her for any therapy, I would consider a nonanthracycline- and
trastuzumab-based regimen.
DR BURSTEIN: She has HER2-overexpressing disease. All of us feel that
trastuzumab is an important treatment for women in this situation. If the
cardiologist clears her for any therapy, I would consider a nonanthracycline- and
trastuzumab-based regimen.
![]() DR LOVE: Which specific chemotherapy regimen would you have used with
trastuzumab?
DR LOVE: Which specific chemotherapy regimen would you have used with
trastuzumab?
![]() DR BURSTEIN: The timing of this patient’s diagnosis was somewhere between
the ASCO 2005 presentation of three adjuvant trastuzumab trials and Dennis’s
presentation of the BCIRG 006 data at the 2005 San Antonio Breast Cancer
Symposium. In fall 2005, you had the HERA data. In HERA, some women
received nonanthracycline-based regimens, and trastuzumab was safely administered
after chemotherapy (Piccart-Gebhart 2005; Smith 2007).
DR BURSTEIN: The timing of this patient’s diagnosis was somewhere between
the ASCO 2005 presentation of three adjuvant trastuzumab trials and Dennis’s
presentation of the BCIRG 006 data at the 2005 San Antonio Breast Cancer
Symposium. In fall 2005, you had the HERA data. In HERA, some women
received nonanthracycline-based regimens, and trastuzumab was safely administered
after chemotherapy (Piccart-Gebhart 2005; Smith 2007).
You could have considered a CMF-type of program followed by trastuzumab. Nowadays, I believe we would use something like the TCH program Dennis and his colleagues put together.
![]() DR LOVE: Dennis, what would you do with this patient?
DR LOVE: Dennis, what would you do with this patient?
![]() DR SLAMON: I would use a nonanthracycline-based regimen, based on what
I’ve heard. Her chances of doing well would be as good as with an anthracycline-based regimen without compromising cardiac function.
DR SLAMON: I would use a nonanthracycline-based regimen, based on what
I’ve heard. Her chances of doing well would be as good as with an anthracycline-based regimen without compromising cardiac function.
![]() DR LOVE: Richard, are you comfortable treating her with trastuzumab?
DR LOVE: Richard, are you comfortable treating her with trastuzumab?
![]() DR STEINGART: The hearts of people with aortic stenosis/aortic regurgitation
are stressed and fibrotic. If the pathophysiologic mechanism of trastuzumab
involves interfering with repair mechanisms — these hearts are constantly
stressed and being repaired. I don’t have systematic data, but I do have
anecdotal experience in patients with valvular heart disease who have received
trastuzumab with dramatically abnormal ventricular responses.
DR STEINGART: The hearts of people with aortic stenosis/aortic regurgitation
are stressed and fibrotic. If the pathophysiologic mechanism of trastuzumab
involves interfering with repair mechanisms — these hearts are constantly
stressed and being repaired. I don’t have systematic data, but I do have
anecdotal experience in patients with valvular heart disease who have received
trastuzumab with dramatically abnormal ventricular responses.
Track 21
![]() DR LOVE: Dr De Fusco, what treatment did the patient receive?
DR LOVE: Dr De Fusco, what treatment did the patient receive?
![]() DR DE FUSCO: Being that it was August 2005, not December 2005, and I had
the cardiologist’s assurance that she could tolerate it, we proceeded with AC.
We used dose-dense AC, and she did have some complications of dehydration
and fatigue. She became a little anemic and received growth factor support.
She had some hypotension and tachycardia in her third and fourth cycles.
DR DE FUSCO: Being that it was August 2005, not December 2005, and I had
the cardiologist’s assurance that she could tolerate it, we proceeded with AC.
We used dose-dense AC, and she did have some complications of dehydration
and fatigue. She became a little anemic and received growth factor support.
She had some hypotension and tachycardia in her third and fourth cycles.
At the completion of AC, her ejection fraction was 60 percent by ECHO, and she had some lower-extremity edema. She also had tachycardia. The aortic valve area was the same.
![]() DR LOVE: Dr Steingart, are you still comfortable going ahead with trastuzumab?
DR LOVE: Dr Steingart, are you still comfortable going ahead with trastuzumab?
![]() DR STEINGART: No. I would obtain a BNP (B-type natriuretic peptide). I
would start wondering why this woman had tachycardia and would look for
occult congestive heart failure, despite the normal ejection fraction.
DR STEINGART: No. I would obtain a BNP (B-type natriuretic peptide). I
would start wondering why this woman had tachycardia and would look for
occult congestive heart failure, despite the normal ejection fraction.
![]() DR LOVE: Dr De Fusco, what happened?
DR LOVE: Dr De Fusco, what happened?
![]() DR DE FUSCO: This woman wanted to be as aggressive as possible. So she
went ahead with paclitaxel in January. I didn’t start trastuzumab until the third
dose of paclitaxel because I wanted her to be further out from the AC.
DR DE FUSCO: This woman wanted to be as aggressive as possible. So she
went ahead with paclitaxel in January. I didn’t start trastuzumab until the third
dose of paclitaxel because I wanted her to be further out from the AC.
She was using diuretics, and in March she started developing some increasing dyspnea and intermittent wheezing. Her eleventh dose of trastuzumab was administered in April. I talked about stopping trastuzumab and couldn’t get the patient to agree as long as the cardiologist was working with her and treating her.
An ECHO in April showed an ejection fraction of 45 percent. She was admitted in May for a cardiac catheterization, and she had 20 percent obstruction of the left circumflex and the left anterior descending (LAD) artery. Her aortic valve area had now dropped. Her ejection fraction had also decreased to 35 percent.
It was urgently planned that she have an aortic valve replacement. She then had a cardiac arrest in the hospital. She came out of it with minimal neurologic defect, other than some amnesia for the event. She underwent aortic valve replacement with reconstruction of the aortic route.
Her ECHO in June showed an LVEF of 55 percent, and she had ongoing problems with pleural effusions. In September, the ECHO showed an ejection fraction of 65 percent. She said, “Can’t I have more trastuzumab?” I said, “No.”
Tracks 23-25
![]() DR LOVE: Dennis, have you seen patients develop acute heart failure after
one dose of trastuzumab?
DR LOVE: Dennis, have you seen patients develop acute heart failure after
one dose of trastuzumab?
![]() DR SLAMON: No.
DR SLAMON: No.
![]() DR LOVE: Hal?
DR LOVE: Hal?
![]() DR BURSTEIN: I haven’t seen that. It’s coincidental that it occurred right after
the trastuzumab with paclitaxel, but it would not be inconsistent with anthracycline-induced cardiomyopathy, which usually occurs in the first four to 12
weeks after anthracycline exposure.
DR BURSTEIN: I haven’t seen that. It’s coincidental that it occurred right after
the trastuzumab with paclitaxel, but it would not be inconsistent with anthracycline-induced cardiomyopathy, which usually occurs in the first four to 12
weeks after anthracycline exposure.
![]() DR LOVE: Dr Steingart, do you have any comment on what was happening
with this patient from a cardiology point of view?
DR LOVE: Dr Steingart, do you have any comment on what was happening
with this patient from a cardiology point of view?
![]() DR STEINGART: From time to time we see this dramatic change in the
ejection fraction, not with trastuzumab, but with other highly effective cancer
therapies. In other circumstances, I believe the cause of the severe left ventricular
dysfunction is multifactorial and relates to cytokines.
DR STEINGART: From time to time we see this dramatic change in the
ejection fraction, not with trastuzumab, but with other highly effective cancer
therapies. In other circumstances, I believe the cause of the severe left ventricular
dysfunction is multifactorial and relates to cytokines.
We see it with huge increases in white counts, effective debulking therapies in ovarian cancer and tumor lysis syndromes. I’m told it’s a sign of an effective antitumor agent. It is conceivable that it is an effect of tissue factors influencing left ventricular function and not necessarily a direct toxic effect of the drug on the heart.
We work very hard in the metastatic setting to support the oncologist in the continued use of trastuzumab with particular attention to symptomatic status and quality-of-life issues. We balance heart failure risk and effectiveness of trastuzumab. In the metastatic setting, the thresholds are different for withholding trastuzumab.