
 |
||||||||

| Tracks 1-6 | ||||||||||||||
|
Select Excerpts from the Interview
Tracks 1-3
![]() DR LOVE: Would you review the background to the data you presented
at the 2007 San Antonio Breast Cancer Symposium evaluating the
Oncotype DX assay for postmenopausal women with ER-positive, node-positive
early breast cancer?
DR LOVE: Would you review the background to the data you presented
at the 2007 San Antonio Breast Cancer Symposium evaluating the
Oncotype DX assay for postmenopausal women with ER-positive, node-positive
early breast cancer?
![]() DR ALBAIN: The Oncotype DX 21-gene assay has been used with increasing
frequency in this country for patients with lymph node-negative breast cancer.
We learned that it had prognostic utility for patients with lymph node-negative
disease who plan on taking five years of tamoxifen (Paik 2004).
DR ALBAIN: The Oncotype DX 21-gene assay has been used with increasing
frequency in this country for patients with lymph node-negative breast cancer.
We learned that it had prognostic utility for patients with lymph node-negative
disease who plan on taking five years of tamoxifen (Paik 2004).
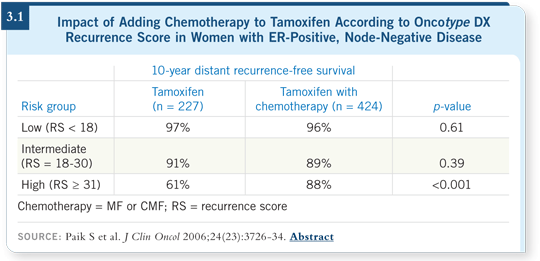
More importantly, it was paradigm shifting in terms of how we thought about the proportional reduction in recurrence achievable by chemotherapy. Instead of a similar degree of benefit across all risk levels, the NSABP reported that the chemotherapy benefit was greatest for patients with a high recurrence score and almost nonexistent for patients with the lowest recurrence scores, with a continuum in between (Paik 2006; [3.1]).
No data for the Oncotype DX assay existed with a no-chemotherapy control arm in a population of patients with lymph node-positive, ER-positive disease.
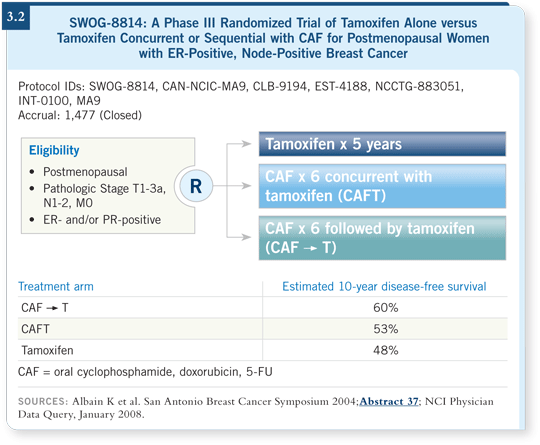
SWOG-8814 was an ideal study to address the question of prognosis in a tamoxifen-alone arm and, more importantly, the prediction of benefit from chemotherapy, which in this case was an anthracycline-based, second-generation regimen (Albain 2004).
SWOG-8814 included the following treatment arms: tamoxifen alone, CAF concurrent with tamoxifen and CAF followed by tamoxifen. CAF followed by tamoxifen was superior to tamoxifen alone in terms of disease-free and overall survival. The outcome for concurrent CAF and tamoxifen was inferior to CAF followed by tamoxifen (Albain 2004; [3.2]).
Thus, for our analysis at the 2007 San Antonio Breast Cancer Symposium, we did not consider the concurrent arm. In our subset from SWOG-8814, the benefit of chemotherapy followed by tamoxifen versus tamoxifen alone mirrored the benefit in the parent trial, with a statistical significance adjusted for nodes (Albain 2007). We felt confident that this was a representative sample.
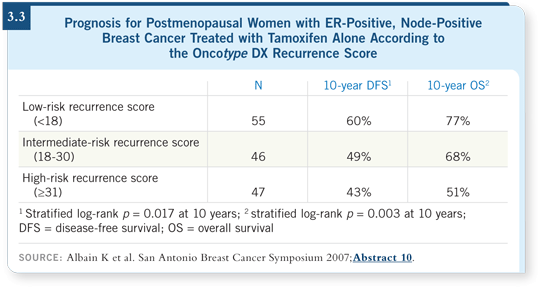
In the tamoxifen-alone arm of SWOG-8814, we saw the same strong prognostic utility of the Oncotype DX recurrence score. Approximately 40 percent of the patients had low recurrence scores and about 32 percent had high recurrence scores. The distribution in this population with node-positive disease was different than in the trials with node-negative disease, as you would expect (Albain 2007).
The curves for low, intermediate and high recurrence scores for the patients with node-negative disease indicate that the group with low recurrence scores had excellent outcomes, so you feel confident treating them with standard endocrine therapy (Paik 2004).
In this trial subset with node-positive disease, our 10-year event rate for the group with low recurrence scores was 40 percent, and that’s not a comfortable level (Albain 2007).
![]() DR LOVE: That seems like a high event rate even for node-positive disease.
DR LOVE: That seems like a high event rate even for node-positive disease.
![]() DR ALBAIN: This was disease-free survival. The Intergroup did not routinely collect data on distant disease or relapse-free intervals, which were collected
by the NSABP. So disease-free survival included events due to cancer, new
primary cancer and death due to other causes. Disease-free survival was 60
percent at 10 years (Albain 2007; [3.3]). Some of these late events may have
been noncompeting causes of death that were included as events.
DR ALBAIN: This was disease-free survival. The Intergroup did not routinely collect data on distant disease or relapse-free intervals, which were collected
by the NSABP. So disease-free survival included events due to cancer, new
primary cancer and death due to other causes. Disease-free survival was 60
percent at 10 years (Albain 2007; [3.3]). Some of these late events may have
been noncompeting causes of death that were included as events.
![]() DR LOVE: What about the patients with high recurrence scores?
DR LOVE: What about the patients with high recurrence scores?
![]() DR ALBAIN: At 10 years, disease-free survival was 43 percent. So the event
rate was 57 percent. We also considered overall survival, and we saw the same
prognostic split. The 10-year overall survival rate for the group with low
recurrence scores was 77 percent, whereas for the group with high recurrence
scores, it was 51 percent (Albain 2007; [3.3]).
DR ALBAIN: At 10 years, disease-free survival was 43 percent. So the event
rate was 57 percent. We also considered overall survival, and we saw the same
prognostic split. The 10-year overall survival rate for the group with low
recurrence scores was 77 percent, whereas for the group with high recurrence
scores, it was 51 percent (Albain 2007; [3.3]).
Track 3
![]() DR LOVE: What about the impact of chemotherapy according to the
Oncotype DX recurrence score?
DR LOVE: What about the impact of chemotherapy according to the
Oncotype DX recurrence score?
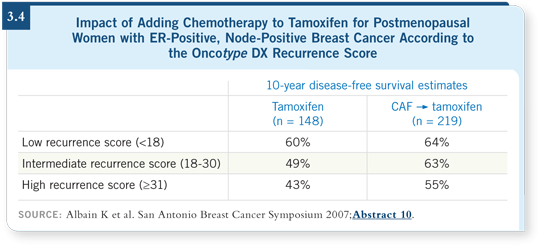
![]() DR ALBAIN: The chemotherapy benefit was strong in the group with high
recurrence scores, whereas it was nonexistent in the group with low recurrence
scores. If you examine the curves carefully, you notice that in the group
with low recurrence scores, the tamoxifen-alone arm tracks above the
CAF
DR ALBAIN: The chemotherapy benefit was strong in the group with high
recurrence scores, whereas it was nonexistent in the group with low recurrence
scores. If you examine the curves carefully, you notice that in the group
with low recurrence scores, the tamoxifen-alone arm tracks above the
CAF![]() tamoxifen arm until the last time point. At 10 years, a 64 percent
disease-free survival rate for CAF
tamoxifen arm until the last time point. At 10 years, a 64 percent
disease-free survival rate for CAF![]() tamoxifen versus 60 percent for tamoxifen
alone is observed (Albain 2007; [3.4]).
tamoxifen versus 60 percent for tamoxifen
alone is observed (Albain 2007; [3.4]).
In the group with high recurrence scores, the disease-free survival at 10 years
with tamoxifen alone is 43 percent, and it’s 55 percent with CAF![]() tamoxifen,
which is a 12 percent absolute difference (Albain 2007; [3.4]).
tamoxifen,
which is a 12 percent absolute difference (Albain 2007; [3.4]).
Track 4
![]() DR LOVE: Putting aside reimbursement issues, do you think a role
exists for the Oncotype DX assay among patients with node-positive
breast cancer?
DR LOVE: Putting aside reimbursement issues, do you think a role
exists for the Oncotype DX assay among patients with node-positive
breast cancer?
![]() DR ALBAIN: Yes, I do. We need to start using it and learn how it’s helping.
We plan to conduct a prospective trial, but we’re 10 to 15 years from an
answer. If we can get around issues of reimbursement, which are not insignificant,
I want to start using this test in the scenarios in which the standard
pathology report leads me to believe patients may have a low recurrence score.
DR ALBAIN: Yes, I do. We need to start using it and learn how it’s helping.
We plan to conduct a prospective trial, but we’re 10 to 15 years from an
answer. If we can get around issues of reimbursement, which are not insignificant,
I want to start using this test in the scenarios in which the standard
pathology report leads me to believe patients may have a low recurrence score.
![]() DR LOVE: From the beginning, people were talking about using the Oncotype
DX assay in node-negative disease when the patient or doctor was on the fence
about chemotherapy as opposed to a situation in which the patient wanted or
didn’t want chemotherapy. A similar guideline might relate to patients with
node-positive disease.
DR LOVE: From the beginning, people were talking about using the Oncotype
DX assay in node-negative disease when the patient or doctor was on the fence
about chemotherapy as opposed to a situation in which the patient wanted or
didn’t want chemotherapy. A similar guideline might relate to patients with
node-positive disease.
When you decide, based on the age or the number of nodes, that you will definitely use chemotherapy, perhaps it will not be relevant. Or perhaps the patient’s comorbidities are so extensive that there is no way you would even consider it.
![]() DR ALBAIN: Yes, but I’m also going to start ordering it when I think I know
the answer. We presented at ASCO 2007 a multicenter study for women
with node-negative disease of the impact of the Oncotype DX assay results on
prospective decision-making by doctors and patients.
DR ALBAIN: Yes, but I’m also going to start ordering it when I think I know
the answer. We presented at ASCO 2007 a multicenter study for women
with node-negative disease of the impact of the Oncotype DX assay results on
prospective decision-making by doctors and patients.
When you thought you knew what you were doing, you changed your mind in a third of the cases, either to use therapy when you hadn’t expected to or vice versa (Lo 2007).
I’m not ready to say it will only be used when the patient and/or doctor are undecided about the use of chemotherapy, but those may be the types of patients with node-positive disease that we should start with.
In other words, that woman with 15 positive nodes is not someone for whom I will order a recurrence score assay right off the bat.
EDITOR'S NOTE
San Antonio adventure, 2007
Neil Love, MD
- Select publications
INTERVIEWS
Stephen E Jones, MD
- Select publications
Sir Richard Peto, FRS
- Select publications
Kathy S Albain, MD
- Select publications
Roundtable Discussion
- Select publications
Joyce O’Shaughnessy, MD
- Select publications
Nancy U Lin, MD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity