
 |
||||||||

| Tracks 1-21 | ||||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 5-6
![]() DR LOVE: What is known from the adjuvant trastuzumab trials about
brain metastases and patterns of relapse?
DR LOVE: What is known from the adjuvant trastuzumab trials about
brain metastases and patterns of relapse?
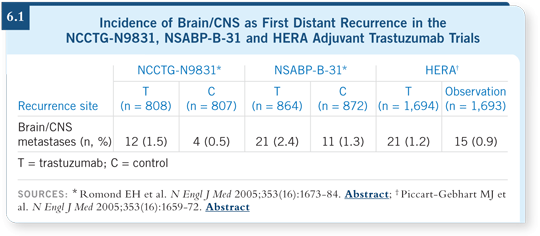
![]() DR LIN: The HERA trial and the joint analysis of NCCTG-N9831 and
NSABP-B-31 have reported the incidence of CNS recurrences separately from other distant first-site recurrences. No decrease in the risk of CNS relapse
is apparent with the use of adjuvant trastuzumab (Romond 2005; Piccart-Gebhart 2005; [6.1]). If anything, there was a trend toward an increased
number of CNS relapses as first event. In comparison, when any first distant
recurrences are considered, those are clearly reduced with trastuzumab.
DR LIN: The HERA trial and the joint analysis of NCCTG-N9831 and
NSABP-B-31 have reported the incidence of CNS recurrences separately from other distant first-site recurrences. No decrease in the risk of CNS relapse
is apparent with the use of adjuvant trastuzumab (Romond 2005; Piccart-Gebhart 2005; [6.1]). If anything, there was a trend toward an increased
number of CNS relapses as first event. In comparison, when any first distant
recurrences are considered, those are clearly reduced with trastuzumab.
![]() DR LOVE: What proportion of patients relapsed with brain-only metastasis?
DR LOVE: What proportion of patients relapsed with brain-only metastasis?
![]() DR LIN: The range across those three studies is one to two percent at the
most. If you evaluate subsequent sites of relapse, in fact, you do not find any
apparent trend toward an increase in CNS relapse. I believe those two pieces
of data together point to the CNS as a sanctuary site.
DR LIN: The range across those three studies is one to two percent at the
most. If you evaluate subsequent sites of relapse, in fact, you do not find any
apparent trend toward an increase in CNS relapse. I believe those two pieces
of data together point to the CNS as a sanctuary site.
![]() DR LOVE: Is that because of the blood-brain barrier, or maybe I should say
the blood-tumor barrier?
DR LOVE: Is that because of the blood-brain barrier, or maybe I should say
the blood-tumor barrier?
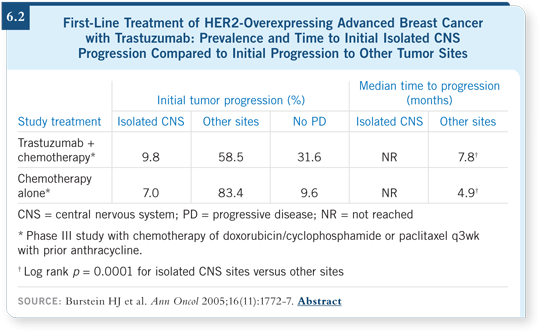
![]() DR LIN: Yes, and I believe trastuzumab is a particularly large molecule and
probably doesn’t penetrate well. Dr Burstein evaluated CNS relapse and CNS
progression versus non-CNS progression among patients receiving first-line
trastuzumab-containing chemotherapy. This analysis revealed that approximately
10 percent of patients in the first-line setting experienced isolated CNS
progression at a time when their non-CNS disease was completely quiescent
(Burstein 2005; [6.2]).
DR LIN: Yes, and I believe trastuzumab is a particularly large molecule and
probably doesn’t penetrate well. Dr Burstein evaluated CNS relapse and CNS
progression versus non-CNS progression among patients receiving first-line
trastuzumab-containing chemotherapy. This analysis revealed that approximately
10 percent of patients in the first-line setting experienced isolated CNS
progression at a time when their non-CNS disease was completely quiescent
(Burstein 2005; [6.2]).
![]() DR LOVE: Were the numbers similar for patients treated with trastuzumab
versus those not receiving trastuzumab?
DR LOVE: Were the numbers similar for patients treated with trastuzumab
versus those not receiving trastuzumab?
![]() DR LIN: Separation occurred only among the patients who received trastuzumab.
If you evaluate time to CNS progression versus time to non-CNS
progression, you see a big separation in the curves. The patients who relapsed
in the CNS experienced the recurrence much later and with control of their
systemic disease, and that effect was recorded among the patients who received
trastuzumab.
DR LIN: Separation occurred only among the patients who received trastuzumab.
If you evaluate time to CNS progression versus time to non-CNS
progression, you see a big separation in the curves. The patients who relapsed
in the CNS experienced the recurrence much later and with control of their
systemic disease, and that effect was recorded among the patients who received
trastuzumab.
![]() DR LOVE: Again suggesting a sanctuary phenomenon?
DR LOVE: Again suggesting a sanctuary phenomenon?
![]() DR LIN: Yes. The data on trastuzumab levels in the cerebrospinal fluid (CSF)
are limited, and it does not seem to penetrate the CSF well. A recent paper reported testing CSF soon after patients received cranial radiation therapy to
ascertain whether radiation therapy makes the blood-brain barrier more leaky
(Stemmler 2007). Trastuzumab penetration was improved, but only slightly.
DR LIN: Yes. The data on trastuzumab levels in the cerebrospinal fluid (CSF)
are limited, and it does not seem to penetrate the CSF well. A recent paper reported testing CSF soon after patients received cranial radiation therapy to
ascertain whether radiation therapy makes the blood-brain barrier more leaky
(Stemmler 2007). Trastuzumab penetration was improved, but only slightly.
![]() DR LOVE: What were the clinical research implications of these data sets?
DR LOVE: What were the clinical research implications of these data sets?
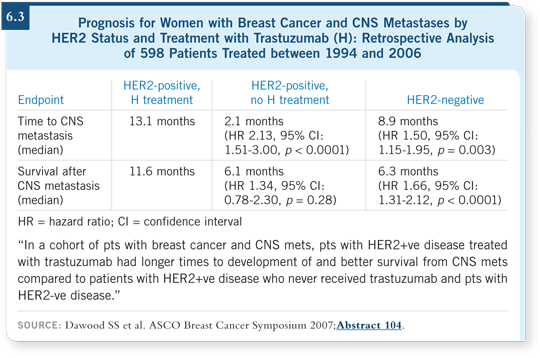
![]() DR LIN: We know that about a third of women with HER2-positive disease
ultimately develop clinically evident, symptomatic CNS metastases. If you
evaluate the prognosis of patients with CNS metastases in the pretrastuzumab
era versus the post-trastuzumab era, you observe a clear difference.
DR LIN: We know that about a third of women with HER2-positive disease
ultimately develop clinically evident, symptomatic CNS metastases. If you
evaluate the prognosis of patients with CNS metastases in the pretrastuzumab
era versus the post-trastuzumab era, you observe a clear difference.
The MD Anderson investigators conducted a retrospective series in which they evaluated survival time from CNS diagnosis for patients with either HER2-positive or HER2-negative disease (Pinder 2007). All the patients fared poorly, but the patients with HER2-positive disease fared particularly poorly.
MD Anderson presented an analysis of a cohort of patients from the posttrastuzumab era, in which patients with HER2-positive disease fared better. The median survival after CNS diagnosis was approximately two years, which is extraordinary for this population (Dawood 2007; [6.3]). I believe we can attribute that to the fact that people are no longer dying from liver metastases.
Track 8
![]() DR LOVE: What do we know about lapatinib and the brain?
DR LOVE: What do we know about lapatinib and the brain?
![]() DR LIN: Initial studies evaluated structurally related compounds, such as
gefitinib or erlotinib, in patients with non-small cell lung cancer and CNS
disease, and a series of case reports led to our pilot Phase II study evaluating
the role of lapatinib for women with brain metastases from HER2-positive
breast cancer (Lin 2006, 2007).
DR LIN: Initial studies evaluated structurally related compounds, such as
gefitinib or erlotinib, in patients with non-small cell lung cancer and CNS
disease, and a series of case reports led to our pilot Phase II study evaluating
the role of lapatinib for women with brain metastases from HER2-positive
breast cancer (Lin 2006, 2007).
Objective RECIST response rates were modest. Some patients experienced volumetric reductions in their CNS tumor burden, and in fact, the one responder according to RECIST was able to remain on the study for over 11 months and showed a dramatic response.
Tracks 13, 15
![]() DR LOVE: Where do you see lapatinib fitting into disease management
right now in the clinical setting?
DR LOVE: Where do you see lapatinib fitting into disease management
right now in the clinical setting?
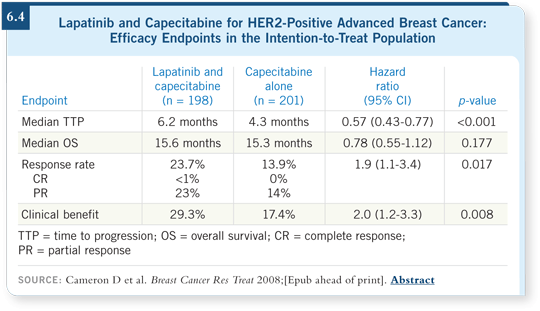
![]() DR LIN: Approval for lapatinib came out of the study by Dr Geyer evaluating
capecitabine with or without lapatinib. The addition of lapatinib improved
time to progression and response rates for patients with HER2-positive
breast cancer after treatment with trastuzumab (Cameron 2008; [6.4]). For
patients whose disease progresses after trastuzumab, switching to lapatinib and
capecitabine is a reasonable option to consider.
DR LIN: Approval for lapatinib came out of the study by Dr Geyer evaluating
capecitabine with or without lapatinib. The addition of lapatinib improved
time to progression and response rates for patients with HER2-positive
breast cancer after treatment with trastuzumab (Cameron 2008; [6.4]). For
patients whose disease progresses after trastuzumab, switching to lapatinib and
capecitabine is a reasonable option to consider.
![]() DR LOVE: What have you seen in terms of side effects and toxicity with
lapatinib or lapatinib/capecitabine?
DR LOVE: What have you seen in terms of side effects and toxicity with
lapatinib or lapatinib/capecitabine?
![]() DR LIN: I believe that the most relevant toxicity is diarrhea. We learned
early on that it is important to gain control of the diarrhea. Patients — often
because we are treating them after progression on trastuzumab — tend
to continue receiving the capecitabine/lapatinib even when they are experiencing
diarrhea because they are afraid of missing a dose: What I would call
“overadherence.” Now we are extremely proactive about educating patients
from the beginning to call us if they have more than two loose bowel
movements a day.
DR LIN: I believe that the most relevant toxicity is diarrhea. We learned
early on that it is important to gain control of the diarrhea. Patients — often
because we are treating them after progression on trastuzumab — tend
to continue receiving the capecitabine/lapatinib even when they are experiencing
diarrhea because they are afraid of missing a dose: What I would call
“overadherence.” Now we are extremely proactive about educating patients
from the beginning to call us if they have more than two loose bowel
movements a day.
![]() DR LOVE: Do you see any other side effects or toxicity from that
regimen (6.5)?
DR LOVE: Do you see any other side effects or toxicity from that
regimen (6.5)?
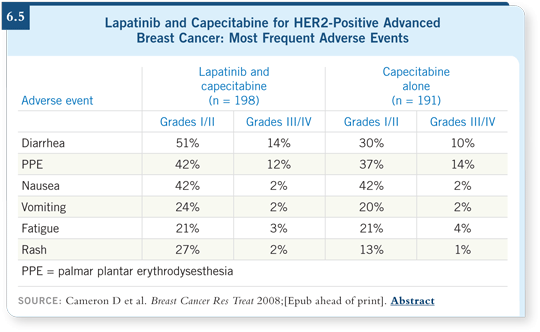
![]() DR LIN: Some people report fatigue or mild nausea, and there is the acneiform
rash that is typical of any of the EGFR inhibitors. Typically it appears over the
lower part of the face and the upper chest.
DR LIN: Some people report fatigue or mild nausea, and there is the acneiform
rash that is typical of any of the EGFR inhibitors. Typically it appears over the
lower part of the face and the upper chest.
Whether it’s responding to treatment or going away on its own is hard to say, but we’ve used topical antibiotics with good results. You definitely see hand-foot syndrome with capecitabine, and this study suggests it may be worse with the addition of lapatinib.
Track 16
![]() DR LOVE: How have the ECOG data with paclitaxel/bevacizumab
affected the treatment of HER2-negative metastatic disease — either in
the ER-negative setting or for patients who are no longer responding to
hormonal therapy?
DR LOVE: How have the ECOG data with paclitaxel/bevacizumab
affected the treatment of HER2-negative metastatic disease — either in
the ER-negative setting or for patients who are no longer responding to
hormonal therapy?
![]() DR LIN: Before the E2100 data were presented (Miller 2007), we used a lot of
first-line capecitabine, as most patients received an anthracycline and a taxane in
the adjuvant setting.
DR LIN: Before the E2100 data were presented (Miller 2007), we used a lot of
first-line capecitabine, as most patients received an anthracycline and a taxane in
the adjuvant setting.
The data indicate that the order in which one administers cytotoxic chemotherapy will probably not effect survival, but when E2100 came we moved toward using a lot of first-line paclitaxel/bevacizumab.
I believe that we were compelled by the significant prolongation of time to progression that was seen in E2100. That benefit was not evident in every patient.
So in the patients who have hormonally sensitive, relatively indolent disease that has progressed through hormonal therapy, I am still comfortable administering first-line capecitabine. I will treat most patients with triple-negative breast cancer and multiple liver metastases with paclitaxel/bevacizumab.
EDITOR'S NOTE
San Antonio adventure, 2007
Neil Love, MD
- Select publications
INTERVIEWS
Stephen E Jones, MD
- Select publications
Sir Richard Peto, FRS
- Select publications
Kathy S Albain, MD
- Select publications
Roundtable Discussion
- Select publications
Joyce O’Shaughnessy, MD
- Select publications
Nancy U Lin, MD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity