
 |
||||||||

| Tracks 1-14 | ||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 3
![]() DR LOVE: In the last year in our Patterns of Care surveys, we’ve seen more
use of TC (docetaxel/cyclophosphamide) both by investigators and oncologists
in practice. What are your thoughts about this regimen (Jones 2007)?
DR LOVE: In the last year in our Patterns of Care surveys, we’ve seen more
use of TC (docetaxel/cyclophosphamide) both by investigators and oncologists
in practice. What are your thoughts about this regimen (Jones 2007)?
![]() DR O’SHAUGHNESSY: Interestingly, TC versus AC shows about the same
improvement in outcome that TAC versus FAC shows. With TAC versus FAC,
the hazard ratio is 0.72 (Martin 2003). In Steve Jones’s trial of TC or AC, it is
0.67 (Jones 2006).
DR O’SHAUGHNESSY: Interestingly, TC versus AC shows about the same
improvement in outcome that TAC versus FAC shows. With TAC versus FAC,
the hazard ratio is 0.72 (Martin 2003). In Steve Jones’s trial of TC or AC, it is
0.67 (Jones 2006).
People aren’t wrong to use TC for higher-risk, node-positive disease, but the question of duration remains for patients with higher nodal burden and presumably higher micrometastatic burden. The question is whether four cycles are enough, so most of us will err on the side of six or eight. I use TC all the time in cases for which I used to use AC, which were the patients at lower risk, such as those with ER-positive, node-negative disease or the patients with tiny, triple-negative disease, who will gain that one to four percent absolute benefit from chemotherapy.
Track 4
![]() DR LOVE: Where do you see things headed with nab paclitaxel?
DR LOVE: Where do you see things headed with nab paclitaxel?
![]() DR O’SHAUGHNESSY: Bill Gradishar’s randomized Phase II trial, with weekly
nab paclitaxel appearing considerably better than the docetaxel at 100 mg/m2 (Gradishar 2007; [4.2]), makes me wonder whether the opportunity exists to
substitute nab paclitaxel for docetaxel in the TC regimen.
DR O’SHAUGHNESSY: Bill Gradishar’s randomized Phase II trial, with weekly
nab paclitaxel appearing considerably better than the docetaxel at 100 mg/m2 (Gradishar 2007; [4.2]), makes me wonder whether the opportunity exists to
substitute nab paclitaxel for docetaxel in the TC regimen.
The nadir with cyclophosphamide occurs around day seven, which is early, so the feasibility must be evaluated. Considering it is not myelosuppressive, nab paclitaxel should be tolerated when administered on day eight and day 15. Evaluating weekly nab paclitaxel/cyclophosphamide versus TC would be a reasonable follow-up to Bill Gradishar’s trial.
Track 6
![]() DR LOVE: What are your thoughts on quality of life with nab paclitaxel
versus paclitaxel versus docetaxel?
DR LOVE: What are your thoughts on quality of life with nab paclitaxel
versus paclitaxel versus docetaxel?
![]() DR O’SHAUGHNESSY: I believe weekly nab paclitaxel is less neurotoxic than
weekly paclitaxel. Nab paclitaxel is virtually nonmyelosuppressive, whereas
docetaxel is myelosuppressive even if you administer a moderate dose of 75 or
85 mg/m2 in the metastatic setting. With docetaxel, eventually you are limited
by fluid retention. Nab paclitaxel unquestionably offers the advantage in the
palliative setting for minimizing side effects. I can’t recall a single patient who
has experienced that lingering, painful neuropathy with nab paclitaxel that I’m
used to seeing with paclitaxel.
DR O’SHAUGHNESSY: I believe weekly nab paclitaxel is less neurotoxic than
weekly paclitaxel. Nab paclitaxel is virtually nonmyelosuppressive, whereas
docetaxel is myelosuppressive even if you administer a moderate dose of 75 or
85 mg/m2 in the metastatic setting. With docetaxel, eventually you are limited
by fluid retention. Nab paclitaxel unquestionably offers the advantage in the
palliative setting for minimizing side effects. I can’t recall a single patient who
has experienced that lingering, painful neuropathy with nab paclitaxel that I’m
used to seeing with paclitaxel.
Track 7
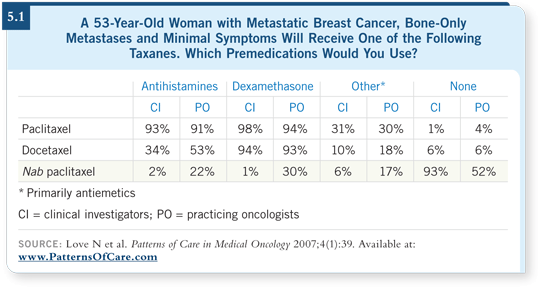
![]() DR LOVE: We’ve seen now in two consecutive Patterns of Care surveys
that in breast cancer, although investigators simply do not use steroids
with nab paclitaxel, one quarter to one third of practicing oncologists are
using corticosteroids with nab paclitaxel (5.1).
DR LOVE: We’ve seen now in two consecutive Patterns of Care surveys
that in breast cancer, although investigators simply do not use steroids
with nab paclitaxel, one quarter to one third of practicing oncologists are
using corticosteroids with nab paclitaxel (5.1).
![]() DR O’SHAUGHNESSY: Wow! One of the main advantages of the drug is that
you don’t need steroids. Steroids weren’t used in the nab paclitaxel trials, and an
increasing body of anecdotal evidence suggests that patients who suffer reactions
with paclitaxel or docetaxel can receive nab paclitaxel without having anaphylactoid
problems. I don’t know of any reason to administer steroids to them.
DR O’SHAUGHNESSY: Wow! One of the main advantages of the drug is that
you don’t need steroids. Steroids weren’t used in the nab paclitaxel trials, and an
increasing body of anecdotal evidence suggests that patients who suffer reactions
with paclitaxel or docetaxel can receive nab paclitaxel without having anaphylactoid
problems. I don’t know of any reason to administer steroids to them.
Track 8
![]() DR LOVE: What are some of the current clinical trials evaluating
nab paclitaxel?
DR LOVE: What are some of the current clinical trials evaluating
nab paclitaxel?
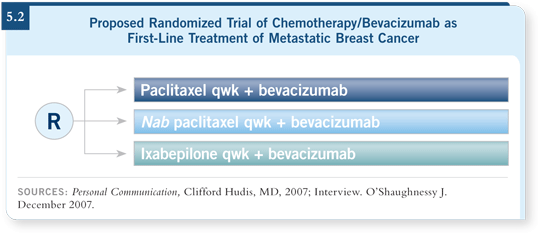
![]() DR O’SHAUGHNESSY: In the metastatic setting, we have the three-arm,
front-line randomized trial that Hope Rugo is heading (5.2). The worldwide
ABIDE trial is a direct comparison to confirm that nab paclitaxel is superior to
docetaxel at 100 mg/m2 because so many people worldwide still like docetaxel.
That is an important trial. Data will be presented at ASCO with nab paclitaxel
at 130 mg/m2 three weeks on, one week off, with bevacizumab at 10 mg/kg
every two weeks. That’s a Phase II, front-line, multicenter trial.
DR O’SHAUGHNESSY: In the metastatic setting, we have the three-arm,
front-line randomized trial that Hope Rugo is heading (5.2). The worldwide
ABIDE trial is a direct comparison to confirm that nab paclitaxel is superior to
docetaxel at 100 mg/m2 because so many people worldwide still like docetaxel.
That is an important trial. Data will be presented at ASCO with nab paclitaxel
at 130 mg/m2 three weeks on, one week off, with bevacizumab at 10 mg/kg
every two weeks. That’s a Phase II, front-line, multicenter trial.
![]() DR LOVE: We see some people using nab paclitaxel and bevacizumab
together. Do you use that combination?
DR LOVE: We see some people using nab paclitaxel and bevacizumab
together. Do you use that combination?
![]() DR O’SHAUGHNESSY: I do. We have Kathy Miller’s Phase III data with
bevacizumab and regular paclitaxel (Miller 2007). The Phase II experience
with nab paclitaxel appears to be reasonable so far.
DR O’SHAUGHNESSY: I do. We have Kathy Miller’s Phase III data with
bevacizumab and regular paclitaxel (Miller 2007). The Phase II experience
with nab paclitaxel appears to be reasonable so far.
![]() DR LOVE: What about nab paclitaxel and trastuzumab?
DR LOVE: What about nab paclitaxel and trastuzumab?
![]() DR O’SHAUGHNESSY: Memorial Sloan-Kettering is evaluating nab paclitaxel/carboplatin and trastuzumab in a Phase II study. Smaller Phase II studies have
been conducted of nab paclitaxel and trastuzumab (Bernstein 2006). No issues
have emerged with it at all, so I believe that’s also reasonable.
DR O’SHAUGHNESSY: Memorial Sloan-Kettering is evaluating nab paclitaxel/carboplatin and trastuzumab in a Phase II study. Smaller Phase II studies have
been conducted of nab paclitaxel and trastuzumab (Bernstein 2006). No issues
have emerged with it at all, so I believe that’s also reasonable.
Track 9
![]() DR LOVE: What is your approach to metastatic visceral disease in the
first-line setting for the patient who previously received an anthracycline
and a taxane?
DR LOVE: What is your approach to metastatic visceral disease in the
first-line setting for the patient who previously received an anthracycline
and a taxane?
![]() DR O’SHAUGHNESSY: When somebody needs a response on the first line,
I turn to a bevacizumab regimen. I want to use it up front, when the safety
is the best, and I want to obtain that prolonged progression-free survival.
My choices are paclitaxel or nab paclitaxel. I don’t have a strong preference
between the two. All things being equal, I’d probably use nab paclitaxel,
with the idea of trying to provide a longer run on the taxane before getting
into toxicity. I would do that if the patient were chemotherapy naïve, if she’d
recently received or if she never received an anthracycline in the adjuvant
setting or if she’d received TC in the adjuvant setting.
DR O’SHAUGHNESSY: When somebody needs a response on the first line,
I turn to a bevacizumab regimen. I want to use it up front, when the safety
is the best, and I want to obtain that prolonged progression-free survival.
My choices are paclitaxel or nab paclitaxel. I don’t have a strong preference
between the two. All things being equal, I’d probably use nab paclitaxel,
with the idea of trying to provide a longer run on the taxane before getting
into toxicity. I would do that if the patient were chemotherapy naïve, if she’d
recently received or if she never received an anthracycline in the adjuvant
setting or if she’d received TC in the adjuvant setting.
![]() DR LOVE: I guess this is based on indirect comparison. We do not know what
the antitumor efficacy of an anthracycline with a taxane would be versus a
taxane with bevacizumab in a patient who is chemotherapy naïve. Yet I hear
from investigators exactly what you said — that they would prefer a taxane
and bevacizumab, for example, to an anthracycline and a taxane. Is that
correct?
DR LOVE: I guess this is based on indirect comparison. We do not know what
the antitumor efficacy of an anthracycline with a taxane would be versus a
taxane with bevacizumab in a patient who is chemotherapy naïve. Yet I hear
from investigators exactly what you said — that they would prefer a taxane
and bevacizumab, for example, to an anthracycline and a taxane. Is that
correct?
![]() DR O’SHAUGHNESSY: Yes. Almost every time two doublets are compared,
they appear to be similar. To me, it’s comparable to either paclitaxel or
nab paclitaxel with bevacizumab versus a well-tolerated, effective doublet
like gemcitabine/paclitaxel (Albain 2004) or capecitabine/docetaxel
(O’Shaughnessy 2002). I’ve been happy with those regimens over the years,
but these days I want to use the bevacizumab up front.
DR O’SHAUGHNESSY: Yes. Almost every time two doublets are compared,
they appear to be similar. To me, it’s comparable to either paclitaxel or
nab paclitaxel with bevacizumab versus a well-tolerated, effective doublet
like gemcitabine/paclitaxel (Albain 2004) or capecitabine/docetaxel
(O’Shaughnessy 2002). I’ve been happy with those regimens over the years,
but these days I want to use the bevacizumab up front.
Track 14
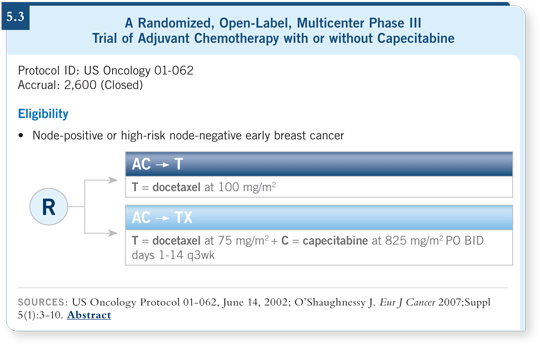
![]() DR LOVE: Can you provide an update on the adjuvant trial of AC
followed by docetaxel with or without capecitabine in node-positive or
higher-risk node-negative breast cancer (5.3)?
DR LOVE: Can you provide an update on the adjuvant trial of AC
followed by docetaxel with or without capecitabine in node-positive or
higher-risk node-negative breast cancer (5.3)?
![]() DR O’SHAUGHNESSY: That trial compared AC followed by docetaxel to AC
followed by docetaxel with capecitabine at a total daily dose of 1,650 mg/m2.
It closed to accrual a couple years ago with 2,610 patients enrolled, and we are
coming up on a median follow-up of nearly three years. The interim analysis
is event driven, not time driven. These patients are faring better. We’ve seen
fewer events than anticipated, so we are waiting.
DR O’SHAUGHNESSY: That trial compared AC followed by docetaxel to AC
followed by docetaxel with capecitabine at a total daily dose of 1,650 mg/m2.
It closed to accrual a couple years ago with 2,610 patients enrolled, and we are
coming up on a median follow-up of nearly three years. The interim analysis
is event driven, not time driven. These patients are faring better. We’ve seen
fewer events than anticipated, so we are waiting.
EDITOR'S NOTE
San Antonio adventure, 2007
Neil Love, MD
- Select publications
INTERVIEWS
Stephen E Jones, MD
- Select publications
Sir Richard Peto, FRS
- Select publications
Kathy S Albain, MD
- Select publications
Roundtable Discussion
- Select publications
Joyce O’Shaughnessy, MD
- Select publications
Nancy U Lin, MD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity