You are here: Home: Special Report: 21st Annual Miami Breast Cancer Conference 2004:
B: HER2 Assessment
| RESEARCH LEADER COMMENTARY |
 |
Importance of accurate HER2 testing
Whenever we have a new therapy requiring a predictive test, how that therapy performs is dependent on how good the test is at identifying the appropriate target. Both the NSABP adjuvant trial and the Intergroup trial indicated that HER2 testing in centers around the country — both community centers and academic centers appeared to be less than perfect. Approximately 25 percent of the time, the test that was done in the local hospital — nonacademic institutions and academic institutions alike — couldn't be confirmed at a central testing site.
We need to be careful about where the HER2 testing is performed and view results from less-experienced labs with caution. This is especially important in the adjuvant setting where, unlike the metastatic setting, we're committing the patient to a course of therapy and we have no way of knowing if the treatment is working.
Also, when we are banking on results from clinical trials, it is critical that we know the testing is accurate. Currently, there's no established adjuvant role for trastuzumab, but I suspect in the next three to five years we'll learn whether it's an effective adjuvant therapy. Then accurate testing will be important to correctly identify the patients who will receive the maximum benefit from therapy.
— Eric P Winer, MD
Every patient with metastatic breast cancer in my practice has her tumor evaluated for HER2 gene amplification by FISH. Tumors with an IHC score of 3+ should be evaluated by FISH because they may not have gene amplification. In those with an IHC score of 0 or 1+, three percent and seven percent, respectively, will have HER2 gene amplification by FISH. We need to determine HER2 status accurately because it is a matter of life or death.
— Melody Cobleigh, MD
Concordance between local and central laboratory HER2 testing
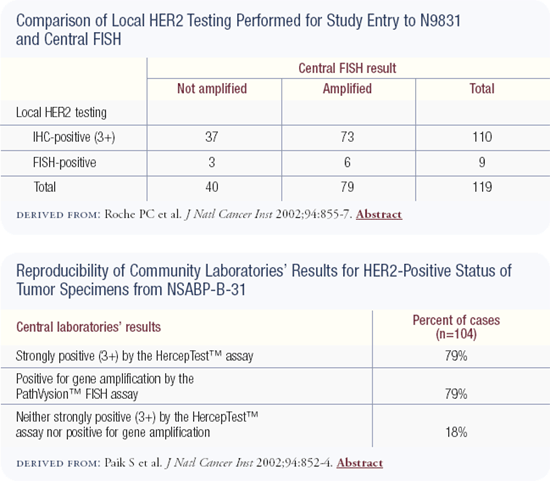
We were surprised when we found poor concordance between community and central laboratory HER2 testing, in terms of both HER2 protein expression and gene amplification. Perhaps more unexpected, we found poor concordance in terms of FISH testing in a central laboratory compared to the local laboratories. This last fact really came as a surprise to us and many others because the prevalent notion regarding FISH was that it was 100 percent accurate.
I've learned about these tests by spending time with our pathologists and looking at exactly what they see under the microscope with FISH. Although, theoretically, it is a matter of counting dots, it's not as simple as that — many tumors are aneuploid, some tumors have deletions of the chromosomes, and some tumors have clumping of dots in one spot. In other specimens it may be difficult to obtain the appropriate hybridization. There are some technical difficulties involved in FISH analysis.
The data from these 119 cases was so important that we actually changed the eligibility criteria for this large cooperative group trial (NCCTG-N9831). We modified the protocol so that physicians can still conduct HER2 testing based on any technology in their local laboratories. The patient is then enrolled in the study and starts the doxorubicin/cyclophosphamide (AC) portion of the chemotherapy.
During that time, we test the tumor specimens again by the HercepTest and the PathVysion FISH assay. If we find that neither of those two tests demonstrates HER2 positivity, we send the tumor specimen to another central laboratory to double-check our laboratory at the Mayo Clinic. If the other central laboratory also finds that the tumor is HER2-negative by both assays, then we notify the physician that the patient really should not participate in the trial.
— Edith A Perez, MD
Algorithm for HER2 testing: IHC versus FISH
Considerable controversy remains regarding the optimal method to routinely evaluate HER2 status. I won't treat a patient with metastatic breast cancer until I have a FISH assay. In the June 2002 issue of the Journal of the National Cancer Institute, the NSABP and the Intergroup published their experiences with HER2 assessment, and it really cast doubt on our quality control for immunohistochemistry. Until the College of American Pathologists does something to resolve this problem of quality control, I continue to use FISH.
— Charles Vogel, MD, FACP
I assume that the tumors with a 3+ score on immunohistochemistry (IHC) are truly HER2-positive, and we do not test them further. An IHC score of 3+ is pretty reliable, as long as it is done at a laboratory that performs a lot of assays. If a tumor has a 2+ score on IHC, we test with fluorescence in situ hybridization (FISH). Even in patients with an IHC score of 0 or 1+ and other features of excessively aggressive disease, we may also do a FISH test.
Both the Intergroup and the NSABP study discovered that smaller community hospitals were overscoring tumors as 3+. Close to 20 percent of the 3+ scores were downstaged when they were reviewed centrally. The Intergroup protocol has now been amended to require that the patients wait for final randomization until there is a central review of their HER2 status.
— Debu Tripathy, MD
If one wants to know whether a patient has the HER2 alteration, one should do FISH testing. One should not do a default IHC, and then do FISH, only if the tumor scores 2+. Using that algorithm, patients without the HER2 alteration will be treated with trastuzumab, and other patients with the HER2 alteration may not be treated. The BCIRG trial we are conducting was designed with FISH as the only criteria for assessing HER2 status. I think the day when FISH testing is the only assay used in the community is coming, and I hope it will be sooner rather than later.
— Dennis Slamon, MD, PhD
Tumors that score 2+ IHC are frequently found to be HER2-negative when tested by FISH. In those patients, I routinely have their tumors retested by FISH. On the other hand, I do not obtain a FISH analysis for tumors that score 3+ on IHC performed at a laboratory where I trust the pathologist.
Since HER2-positive breast cancer has a fairly specific phenotype (i.e., steroid receptor-negative, younger age, early relapse), I will retest those types of patients by FISH if I have a two- to three-year-old IHC score of 0 or 1+. If the patient's tumor is IHC-negative and FISH-positive, I will treat them with trastuzumab despite the fact that we do not have clinical data for that group of patients. Tumors that are FISH-positive are likely to have ample amounts of HER2 receptors on their cell surface.
We lack quality control for both IHC and FISH. This is analogous to the situation encountered with estrogen receptor testing in the mid- to late 1970s. One wonders how many patients died because they did not receive adjuvant tamoxifen as a result of inadequate estrogen receptor testing. If adjuvant trastuzumab provides a benefit like adjuvant tamoxifen, we may encounter the same problem.
— George Sledge, MD
Select publications
|
