You are here: Home: Special Report: 21st Annual Miami Breast Cancer Conference 2004:
G: Sequencing Endocrine Therapy in Metastatic Disease


| RESEARCH LEADER COMMENTARY |
 |
Clinical experience with fulvestrant
I've used a fair amount of fulvestrant, and it's very well-tolerated. We've had some very nice responses to fulvestrant, including one of my patients who was enrolled in the original clinical trial of fulvestrant versus anastrozole. She was on fulvestrant for three and a half years, and now she's on anastrozole. The injections have not been an issue for patients, and most women are very grateful that the side-effect profile is close to nil. I think fulvestrant probably crosses the blood-brain barrier and patients do have hot flashes on it, but in general, they're quite mild.
I am a little disquieted by the fact that it can take three to five months to reach a steady state with fulvestrant. A patient with rapidly progressing disease may not benefit from fulvestrant, but fortunately most women with hormone-responsive breast cancer have relatively indolent disease. I'm very interested in the clinical trial in which they are loading fulvestrant 500 mg every two weeks for a couple of doses and then reducing it to 250 mg monthly. That makes sense to me, so I've been trying to load it a little by giving it every three weeks for several injections in an attempt to raise the levels more quickly.
— Joyce O'Shaughnessy, MD
My patients like fulvestrant because it lets them get on with their activities and maintain their quality of life. In my experience, it has been much more likely to result in stable disease rather than produce measurable responses or complete remissions. However, it has stabilized patients with excellent quality of life for long periods of time without having to change therapy.
It'll be interesting to see the trials that move fulvestrant into the front-line setting. All of the hormonal agents, when they first become available, are used in patients with refractory disease.
— Denise A Yardley, MD
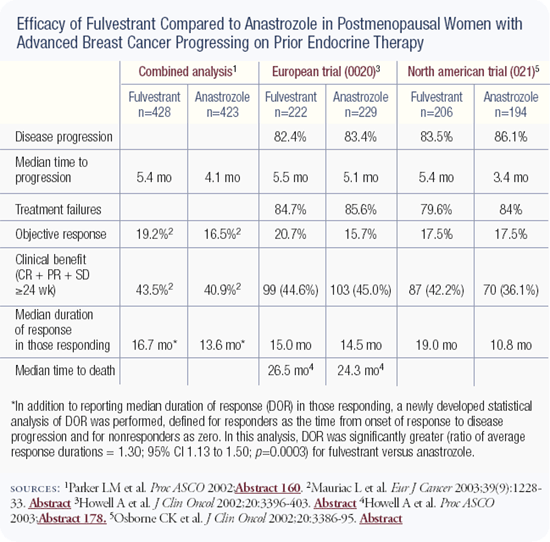
Like many of my colleagues, I'm not quite sure where to use fulvestrant, partly because we have limited clinical trial data. My interpretation of the results of the large North American and European trials is that fulvestrant and anastrozole are roughly equivalent agents in terms of survival.
In the North American trial, fulvestrant appeared to have some advantage over anastrozole in response and time to progression. My approach to therapy is to use survival to guide how I treat patients. The trials didn't demonstrate a survival difference, so I don't feel strongly that one agent is better than the other.
I use fulvestrant regularly in my patients with steroid receptor-positive, metastatic breast cancer. I have patients who prefer receiving an injection once a month to taking pills every day. I have other patients who would prefer a pill to a shot. Aside from the acute discomfort of the injection itself, I've found fulvestrant to be an exceptionally well-tolerated medication.
— George W Sledge, MD
In my clinical experience, fulvestrant is very easy to administer and extremely well-tolerated. My patients have not had any problems with the intramuscular injection. One might assume that a pill is more convenient therapy for a patient than an injection, but that is not necessarily so. Convenience is an individual choice. Some patients would rather receive a shot once a month than take a pill every day.
Fulvestrant has been exceptionally well-tolerated and I've seen responses in heavily pretreated patients. Fulvestrant also works after multiple endocrine failures, including tamoxifen and the aromatase inhibitors, even in a third- or fourth-line setting. We now have a very well-tolerated endocrine agent to add to our armamentarium in the metastatic setting.
— Richard M Elledge, MD
Sequencing hormonal agents in postmenopausal women with metastatic disease
In a postmenopausal woman whose disease relapses on adjuvant tamoxifen, I would use fulvestrant because I've seen some very long remissions with it. I will use an aromatase inhibitor later because data indicate that patients with disease that progresses on fulvestrant can still respond to other endocrine treatments (e.g., aromatase inhibitors and megestrol acetate).
A couple of reports have looked at the response to fulvestrant in patients who have received an aromatase inhibitor. A fairly small Swiss study reported that about one-third of patients derived clinical benefit from fulvestrant after treatment with tamoxifen or an aromatase inhibitor. A compassionate-use study, reported at ASCO 2003, reported about 60 patients with fulvestrant as second-, third- or fourth-line therapy. Fulvestrant had a more than 50 percent clinical benefit rate in those patients.
— Stephen E Jones, MD
Clinical trials of fulvestrant in the metastatic setting: Fulvestrant anastrozole
Fulvestrant has a different mechanism of action than the other hormonal agents because it downregulates both the estrogen and progesterone receptors. It's a well-tolerated parenteral agent — a potential advantage for patients with compliance issues. There is a subset of patients who had an exceptionally long duration of response with fulvestrant, and this is not fully appreciated.
US Oncology participated in one of the trials comparing fulvestrant to anastrozole, and I personally enrolled 27 patients in the study. Five of those patients had responses lasting longer than three years, which is really extraordinary for any endocrine treatment; two of the patients had responses lasting longer than four years. Of those five patients, four have progressed and had their therapy unblinded; all four were on fulvestrant. I would bet the fifth patient, although her treatment remains blinded, is also on fulvestrant.
A reanalysis of the North American and the European fulvestrant trials used a different statistical model called the mean duration of response. In that statistical model, values were assigned to every patient: patients with disease that did not respond were assigned a value of zero and patients with disease that did respond were assigned a number to correspond with the number of months of the response. With those calculations, fulvestrant had a significantly longer duration of response. It was 36 percent longer in the North American trial and 27 percent longer in the European trial.
— Stephen E Jones, MD
The trials of fulvestrant versus anastrozole in patients progressing on tamoxifen were large, well-executed studies — in contrast to other hormone therapy trials done as recently as five years ago. The fulvestrant versus anastrozole trials demonstrated that fulvestrant is a very safe therapeutic agent for cancer. There were virtually no toxicities other than background noise.
The main difference between fulvestrant and anastrozole in the American trial was the increased duration of response in the fulvestrant arm. Not only was there a statistically significant improvement from 10 months to 19 months, but this time difference is clinically and humanly worthwhile in the metastatic setting. It tells us that this agent might give us a bit of a boost in the adjuvant setting.
— Richard M Elledge, MD
I'm concerned that physicians routinely give fulvestrant to patients with hormone receptor-positive metastatic disease who have received multiple chemotherapy regimens and hormonal therapies, and then judge fulvestrant to be a relatively inactive drug. This is probably not a fair evaluation.
In randomized trials of patients receiving fulvestrant or anastrozole in the metastatic setting, fulvestrant was at least as good as anastrozole, and I find the data quite persuasive. The one striking difference that favored fulvestrant was that there were fewer arthralgias and musculoskeletal complaints and, in our institution, the injection has not been a major issue.
— Eric P Winer, MD
Fulvestrant versus tamoxifen
Much to our surprise, the trial comparing fulvestrant versus tamoxifen did not demonstrate that fulvestrant was superior in the first-line setting. Extrapolating what we know from previous trials of fulvestrant versus anastrozole, and of anastrozole versus tamoxifen, we predicted that fulvestrant would be better than tamoxifen. However, in the study we just didn't see it.
Some have suggested that the dose of fulvestrant was inadequate. While I believe this should be explored, I'm not entirely convinced it is the reason. Another possibility relates to the fact that most patients in the second-line study had been treated with tamoxifen or were coming straight off of tamoxifen. This may have somehow altered the phenotype, perhaps causing fulvestrant to work better in the second-line trial, as opposed to treatment-naive tumors or those that have not been recently exposed to tamoxifen. After reviewing the data, the reason the first-line trial didn't demonstrate fulvestrant to be superior to tamoxifen is still not clear.
— Richard M Elledge, MD
Data have been presented demonstrating that fulvestrant is active in the first-line setting, but in the first-line study comparing it to tamoxifen, it did not prove to be more active. The primary endpoint was time to treatment failure, and tamoxifen was superior, although not statistically. One question that has been raised in this setting is whether the fulvestrant dose was adequate.
A number of investigators feel that some of the early failures seen in the comparison of fulvestrant and tamoxifen might indicate that patients were not brought up to their steady-state level, and that a loading dose of fulvestrant may be necessary.
This is currently being studied in a clinical trial that gives patients a loading dose in the first month of therapy. I would not recommend the concept of a loading dose in a nonprotocol setting at this time. We already know that when fulvestrant was compared to anastrozole as treatment for progression after tamoxifen, the current dose was adequate.
— Leroy M Parker, MD
Novel hormonal therapy combinations
There is an increasing body of preclinical evidence suggesting that breast cancers that become resistant to tamoxifen or fulvestrant have upregulation of epidermal growth factor receptor (EGFR) and HER2 expression. As those endocrine-sensitive cells become endocrine-resistant and the EGFR and HER2 upregulate, some of the sensitivity to the endocrine agents may return if those cells are exposed to EGFR inhibitors. Series of trials are being conducted to evaluate the role of fulvestrant or other hormonal agents in combination with gefitinib. ECOG is initiating a Phase II randomized trial comparing fulvestrant/gefitinib to anastrozole/gefitinib.
The combination of an aromatase inhibitor and fulvestrant is of some interest, but the difficulty with such a study is that fulvestrant eliminates the estrogen receptor. Theoretically, if the estrogen receptor is eliminated, then the cells shouldn't care how much estrogen is present.
— Robert W Carlson, MD
Select publications
|
