You are here: Home: Special Report: 21st Annual Miami Breast Cancer Conference 2004:
C: Adjuvant Endocrine Therapy in Postmenopausal Patients: ATAC

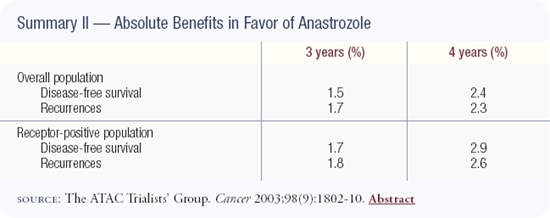
| RESEARCH LEADER COMMENTARY |
 |
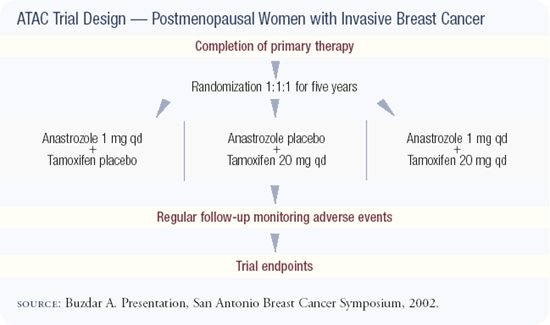
Updated results of the ATAC trial
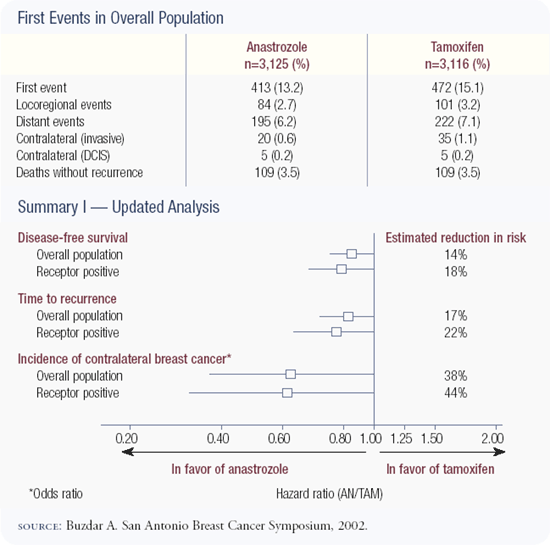
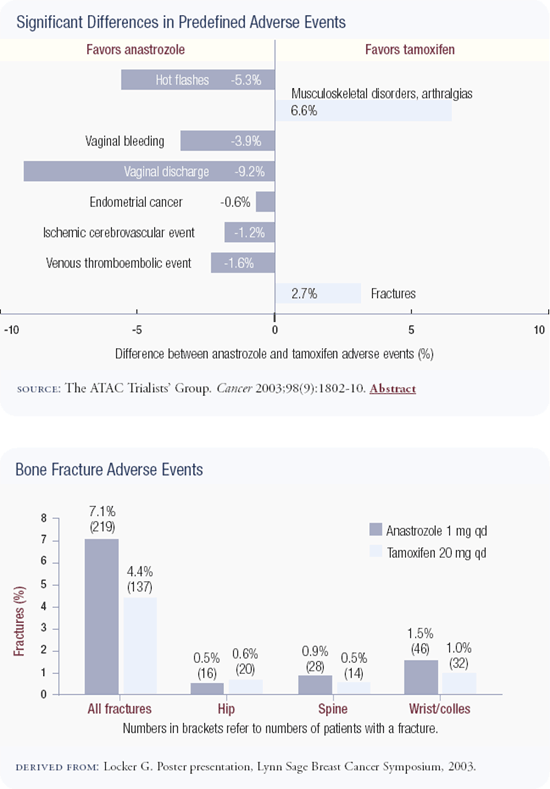
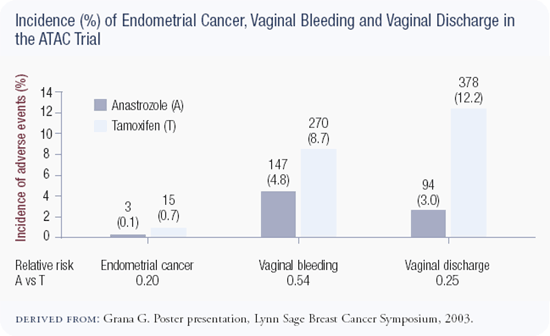
The ATAC trial is a superb study of more than 9,000 patients. An update of the data was presented by Dr Aman Buzdar in San Antonio and showed that at four years follow-up, anastrozole was superior to tamoxifen with respect to disease-free survival and event rates. In addition, anastrozole is a less toxic drug without the risks of endometrial cancer or thromboembolic disease. Anastrozole was associated with an increased risk of fractures, which is important because fractures are a cause of mortality in the United States; we need a lot more information with regard to bone. This statistically powerful trial gives us another option for adjuvant therapy in estrogen receptor-positive postmenopausal patients, and I discuss both tamoxifen and anastrozole with patients.
— Hyman Muss, MD
Now my default therapy for postmenopausal women with estrogen receptor-positive tumors is anastrozole, unless contraindicated. We have another year of follow-up in the ATAC trial, and I am impressed by the separation of the curves. The safety update is also comforting. The fracture rate isn't racing away, the relative risks are stable, and the other safety profile issues continue to strongly favor anastrozole.
— Michael Baum, MD, ChM, FRCS, FRCR
The initial publication of the ATAC results caused concern because the data represented only about two-and-a-half years of follow-up. Now the median follow-up is four years, there are no new safety concerns and the early efficacy advantages have persisted — in fact, the absolute differences are increasing with time. I believe the data provide strong support for the adjuvant use of anastrozole in postmenopausal patients with hormone receptor-positive, early-stage breast cancer.
— Aman Buzdar, MD, FACP
Side effects and toxicities of anastrozole versus tamoxifen
The biggest problem with tamoxifen is not the risk of thromboembolism or uterine cancer, but managing uterine bleeding. Any woman who has uterine bleeding on tamoxifen goes through a panoply of tests, which causes a great deal of anxiety. At some time during their five years of therapy, a large percentage of women undergo a gynecologic procedure as a result of tamoxifen. What's really unacceptable about tamoxifen is that we overinvestigate some of these symptoms. This may be due to our medicolegal milieu, but it contributes to a miserable lifestyle and a lot of anxiety for women on tamoxifen in the adjuvant and preventative settings.
— Gershon Locker, MD
Implications of the ATAC trial in clinical practice
The results of the ATAC trial are quite compelling. Even if you assume for the sake of argument that the curves will come together with further follow-up, the safety profile of anastrozole is still clearly better than that of tamoxifen. I cannot prevent endometrial cancer short of removing the uterus, but I can prevent or treat osteoporosis and fractures. Since the safety profile of anastrozole is better than that of tamoxifen, and it is therapeutically superior, I have a problem not offering anastrozole to my patients — not as a neutral choice, but as a better choice. I discuss with my patients the enormous amount of clinical experience we have with tamoxifen, but if my sister developed breast cancer today, I would certainly recommend anastrozole as opposed to tamoxifen.
— Gabriel N Hortobagyi, MD
The ATAC trial has had a major impact across the country, and we are seeing more adjuvant anastrozole being used. The ATAC trial results must be discussed with patients, and patients should be aware of the two hormonal therapy options. Many factors go into making a decision about hormonal therapy, including the patient's ability to pay for the drug, her feelings and her history of thromboembolic events.
I am more likely to use adjuvant anastrozole in the patient with higher-risk, node-positive disease. The woman with 10 positive nodes needs every percentage point possible to make sure her cancer doesn't recur. I try to encourage those patients to receive anastrozole.
— Stephen E Jones, MD
ASCO Technology Assessment regarding adjuvant aromatase inhibitors
The ASCO Technology Assessment is a superb document, but it needs to be viewed for exactly what it is. A technology assessment looks at a given therapy, attempts to decide whether that therapy has utility in a given clinical situation and determines what the preponderance of data is within that clinical situation. The ASCO Technology Assessment, in both the first and second versions, states that tamoxifen remains the standard adjuvant therapy to which other therapies should be compared.
Interestingly, several members of the ASCO Technology Panel also sit on the NCCN Practice Guidelines Panel. When the NCCN Practice Guidelines Panel looked at this issue, there was no major dissension in considering anastrozole as an option. The difference between groups occurred because of the different processes.
The ASCO Technology Assessment is strictly evidence-based and cannot go beyond the evidence, so there are no extrapolations beyond five years of anastrozole or the 47 months of follow-up.
In the NCCN Practice Guidelines process, we use a methodology called evidence-based consensus. We establish recommendations based on evidence, but we are also able to use expert consensus in situations where the evidence is lacking. Obviously, 10-year data with adjuvant anastrozole are lacking, but we can come up with expectations about what might happen and make recommendations that extrapolate into the unknown.
The NCCN Practice Guidelines are patient-focused, and they look at the various therapies that are available from a patient's perspective. The NCCN Practice Guidelines Panel believes that women should consider the use of anastrozole, although we don't say it should necessarily be used in preference to tamoxifen.
— Robert W Carlson, MD

The ASCO Technology Assessment that does not support the use of adjuvant anastrozole outside a clinical trial is based on fear of the unknown in the face of the single largest clinical trial ever conducted in the adjuvant setting. We have no comparable trial in the history of medical oncology or breast cancer, and there is no other tumor type with so many well-planned clinical trials conducted. We are in a leadership position in oncology, and we can't advocate doing the best trials and then ignore the results of those trials. Every single trial we do brings with it some of the unknown. We have very compelling data about anastrozole from the ATAC trial, in terms of its therapeutic and safety profile superiority. I would be doing a disservice to my patients who are candidates for adjuvant antiaromatase therapy by not presenting the data. I also present tamoxifen as an option.
— Gabriel N Hortobagyi, MD
Clinical use of adjuvant aromatase inhibitors
Currently, I do not recommend the use of aromatase inhibitors other than anastrozole in the adjuvant setting. I recently published a review in Cancer demonstrating differences in the pharmacology and pharmacokinetics among the newer generation of aromatase inhibitors: anastrozole, letrozole and exemestane. Until we have long-term safety and efficacy data on letrozole and exemestane, I don't recommend their use outside of a clinical trial.
Experimental data in mice show possible benefits of exemestane on bone, but this still needs to be proven in patients. In addition, exemestane is a steroidal molecule that, because of its agonistic effect, may have safety issues similar to those associated with tamoxifen. We don't have enough long-term safety or efficacy data, even in metastatic disease, to know whether these androgenic effects will be beneficial or detrimental when exemestane is given to patients for a long period of time.
— Aman Buzdar, MD, FACP
Select publications
|
