You are here: Home: Special Report: 21st Annual Miami Breast Cancer Conference 2004:
I: Adjuvant Endocrine Therapy: Premenopausal Patients

| RESEARCH LEADER COMMENTARY |
 |
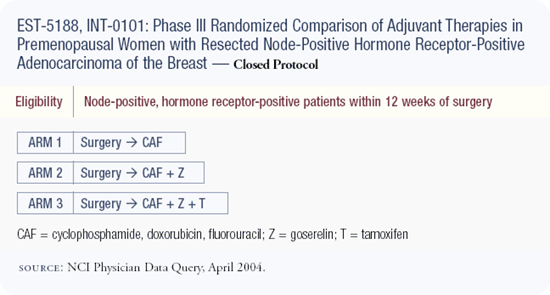
Intergroup Trial 0101
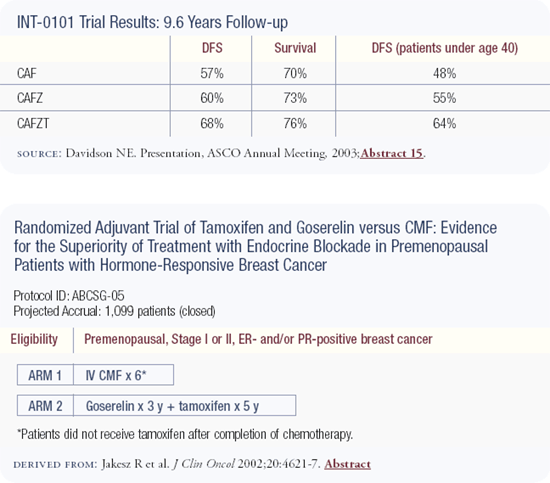
The design of this trial was CAF chemotherapy versus CAF chemotherapy followed by five years of goserelin versus CAF chemotherapy followed by five years of goserelin and tamoxifen. There is no impact on disease-free survival in the overall population with the addition of goserelin, but there is a trend to suggest that the younger patients may benefit.
Although it seemed like such a large clinical trial at the time it was initiated, a study of 1,500 women doesn't have the power to reveal a significant difference even in younger women and even with all this follow-up.
We don't have any new data over the last year but we have a lot of re-examination of old data. My synopsis is that in ER-positive, premenopausal women, tamoxifen is a good drug. Ovarian suppression or ablation is also beneficial, but we are having a difficult time figuring out how to integrate them.
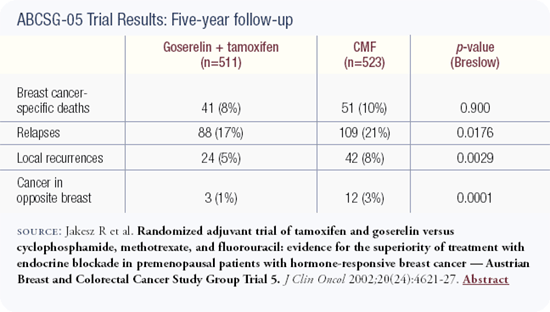
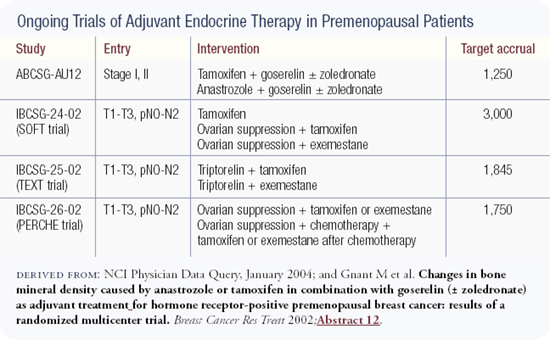
The one new trial that I've seen over the last year is the Austrian trial comparing CMF chemotherapy to ovarian suppression with tamoxifen in premenopausal ER-positive women. They suggested that the outcome was slightly better with the combined endocrine therapy.
In that trial the women who underwent chemotherapy didn't take tamoxifen because it was not the standard of care when the trial was launched. Today we think of that as a pretty profound deficit with that study and related studies, so we need to come together to investigate this further. There is a trio of trials that we are trying to launch worldwide to look at issues of ovarian suppression in young women.
— Nancy E Davidson, MD
SOFT: Ovarian ablation with tamoxifen or an aromatase inhibitor
The adjuvant ovarian suppression trial that I am most enthusiastic about is the Suppression of Ovarian Function Trial (SOFT). Premenopausal ER-positive women who may or may not have received chemotherapy will be randomly assigned to tamoxifen for five years, ovarian suppression/ablation plus tamoxifen, or ovarian suppression/ablation plus an aromatase inhibitor. This very interesting trial will help us address several issues. Does ovarian ablation or suppression add to tamoxifen? And if this is an important strategy, is it better to use tamoxifen or an aromatase inhibitor in women with ovarian suppression? This trial is an international collaboration put together by the International Breast Cancer Study Group (IBCSG).
— Nancy E Davidson, MD
In premenopausal women, there is a rejuvenation of interest in ovarian ablation in combination with tamoxifen. Is ovarian ablation in addition to tamoxifen or in combination with an aromatase inhibitor superior to tamoxifen alone in a premenopausal woman? Right now that is the $64-million question that is being addressed in the SOFT and the Tamoxifen and Exemestane Trial (TEXT).
— G Thomas Budd, MD
The SOFT and TEXT trials are evaluating whether ovarian ablation, with either an aromatase inhibitor or tamoxifen, is beneficial. Right now we just don't know.
— Sandra Swain, MD
ABCSG-12: Adjuvant anastrozole or tamoxifen in combination with (± zoledronic acid) for patients with hormone receptor-positive, premeno-pausal breast cancer
The ABCSG-12 trial has four arms comparing goserelin/tamoxifen to goserelin/ anastrozole with or without zoledronic acid. We included zoledronic acid because it's the most potent bisphosphonate pharmacokinetically, and we were concerned about the risk of osteoporosis with the aromatase inhibitors. Chemotherapy is only permitted as neoadjuvant therapy. No postoperative chemotherapy is allowed.
We did not include a tamoxifen-only arm because we tried to build upon our own results with goserelin/tamoxifen, which is now a national standard in Austria. I also believe tamoxifen-only treatment in premenopausal women is debatable because reasonable evidence indicates that you need to include some cytotoxic treatment.
The early results of ABCSG-12 demonstrate that the combination of goserelin/ anastrozole, and goserelin/tamoxifen to a lesser degree, leads to significant deterioration in bone mineral density in premenopausal women and that this can be completely counteracted by zoledronic acid. Even though tamoxifen has an agonistic effect on bone, when combined with the more potent agent goserelin, it results in a net reduction in bone density. The bone deterioration is more pronounced with anastrozole/goserelin but the difference is not significant at this time. The main message is that zoledronic acid was able to completely prevent bone loss regardless of which hormone combination the patients received.
— Michael F Gnant, MD
Select publications
|
