You are here: Home: Special Report: 21st Annual Miami Breast Cancer Conference 2004:
F: Clinical Trials of Adjuvant Trastuzumab

| RESEARCH LEADER COMMENTARY |
 |
Clinical trials of adjuvant trastuzumab
After the NSABP designed the adjuvant trial B-31, the Intergroup designed a similar trial so that the data could be analyzed together. I think that's great because it will be a stronger analysis. I hope we'll see a benefit with trastuzumab, which has been a miracle drug in the metastatic setting. If this trial is positive, there will still be a lot of scheduling questions to be answered, such as, "How long do you really need trastuzumab, and can it be administered every three weeks rather than weekly?"
— Sandra Swain, MD
The Intergroup adjuvant trial evaluating trastuzumab plus chemotherapy builds on several issues, including the relative importance of anthracyclines in patients with HER2- positive breast cancer, and the value of adjuvant taxanes. Patients randomly assigned to trastuzumab receive it for a year. I believe adjuvant trastuzumab currently should only be used in a clinical trial setting. Clinicians who use this therapy off protocol are essentially shooting in the dark, because we don't understand for how long this therapy should be given, what schedule should be used in combination with chemotherapy, and the potential risks or benefits patients may derive from such treatment. Several major clinical protocols are available, and I hope that every woman diagnosed with HER2-positive breast cancer asks her physician about participation in a clinical trial that will help answer those questions.
— Edith A Perez, MD
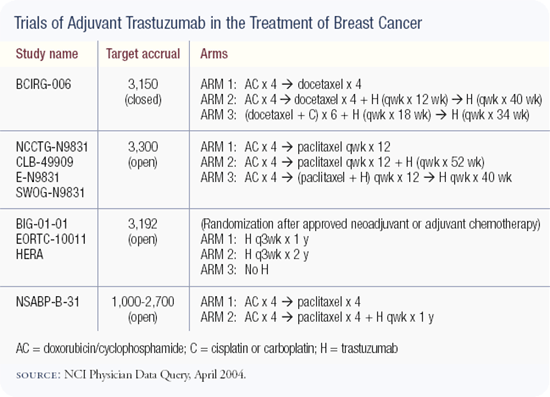
BCIRG-006 is a multinational, randomized, controlled trial for patients with FISH-positive, early-stage breast cancer — either node-positive or high-risk, node-negative disease. Patients are randomized to one of three different treatment arms: AC followed by docetaxel, AC followed by docetaxel/trastuzumab with trastuzumab continued for a total of one year, and trastuzumab/docetaxel with either carboplatin or cisplatin.
For the first time in a large randomized adjuvant study, a non-anthracycline-containing synergistic combination will be put to the test in a very carefully selected patient population. All of the patients must have FISH-positive disease; therefore, I think the trial will define the standard of care for the adjuvant treatment of patients with HER2-positive breast cancer.
The other important component of this trial is safety. There is a data safety monitoring committee and a specific cardiac safety monitoring committee. They are monitoring all of the treatment arms in real time, and they have predefined trigger points that call for an interruption in the protocol if there are any flags for cardiotoxicity in the AC followed by trastuzumab/docetaxel arm.
In fact, the study was designed in such a way that the arm can drop out if we encounter cardiotoxicity problems. We would still have a two-arm study one arm with conventional chemotherapy and the other arm with trastuzumab/platinum/taxane.
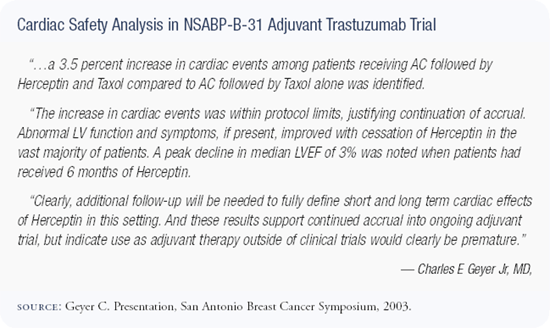
It doesn't appear that cardiac safety is going to be a big issue in the adjuvant trastuzumab trials. Although there was a scare some months ago with the Intergroup trial and one arm was closed temporarily, that arm has reopened and the most recent update, presented by Dr Edith Perez, reveals that the incidence of depressed ejection fractions is the same in all of the arms of the Intergroup trial.
— Mark D Pegram, MD
Nonprotocol use of adjuvant trastuzumab
Trastuzumab is a fabulous drug that has made a huge difference for a lot of patients with metastatic disease and a very poor prognosis. We don't have any efficacy data for adjuvant trastuzumab, so I think it's unwise to use it in that setting outside of a clinical trial. I'm concerned about the potential cardiac toxicity, and we need the studies to mature in order to analyze the toxicity data. On the other hand, there are cases in which I would consider using trastuzumab, such as inflammatory breast cancer, where more of the patients are HER2-positive and survival is poor.
— Sandra Swain, MD
I try not to use trastuzumab in patients with Stage II and IIIA breast cancer outside of a trial, because it's not an established therapy. In patients with inflammatory breast cancer, I don't know that we're ever going to have a randomized study, and at least 50 percent of the time the tumor is HER2-positive. I would be hard-pressed to criticize a physician who wanted to use a trastuzumab-based regimen in a patient with HER2-positive, inflammatory breast cancer.
I feel patients who are eligible for the randomized adjuvant trials should be encouraged to participate. Outside of those trials, I think that the standard adjuvant treatment is a non-trastuzumab-containing combination.
— Eric P Winer, MD
In the nonprotocol adjuvant setting, it's hard to know the right thing to do. I've evaluated patients with high-risk disease — 10 or more positive nodes — in whom I've considered adjuvant trastuzumab therapy off protocol.
I don't want to say that this is something that is widely done at our center — it's infrequent and uncommon. However, the prospects for a patient with that type of disease are really unacceptable. If you consider that trastuzumab prolongs survival in patients with metastatic disease, biologically there are probably many similarities between high-risk Stage II and advanced disease. Therefore, that would be an interesting patient population to study. Off protocol we have considered such patients for adjuvant trastuzumab therapy.
— Mark D Pegram, MD
The research question that has to be answered is: How do we use it appropriately? Do we use AC followed by paclitaxel and concurrent trastuzumab, or should we be using a non-anthracycline-containing regimen to avoid cardiac toxicity? Those two questions are going to be very important to address in clinical trials.
I have not been using trastuzumab in the adjuvant setting but have used it for locally advanced and inflammatory disease. I'm selective in choosing patients for whom I'll use it. Often, it will be the patient who did not respond well to AC or had very aggressive disease.
— Generosa Grana, MD
Select publications
|
