You are here: Home: Special Report: 21st Annual Miami Breast Cancer Conference 2004:
D: Adjuvant Endocrine Therapy in Postmenopausal Patients: Sequencing Tamoxifen and Aromatase Inhibitors

| RESEARCH LEADER COMMENTARY |
 |
Switching adjuvant therapy in clinical practice
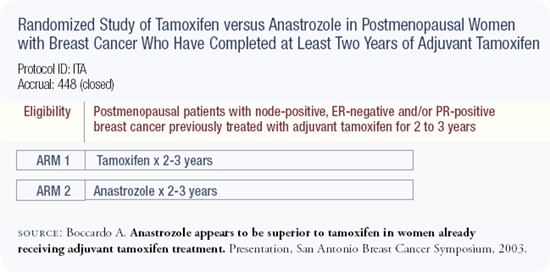
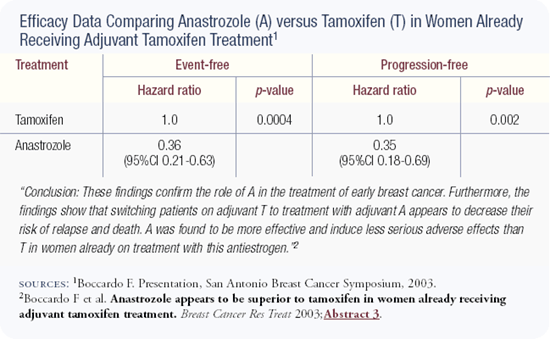
I am usually conservative, especially with my work. The ITA trial is a relatively small trial and the data is still early, so we need to be cautious and avoid over-interpretation of it. However, with that being said, the data speaks for itself and supports an advantage for switching to anastrozole following two to three years of adjuvant tamoxifen. This data also fits in with previous data from a study with aminoglutethimide, the ATAC trial and MA17, all pointing in the same direction.
— Francesco Boccardo, MD
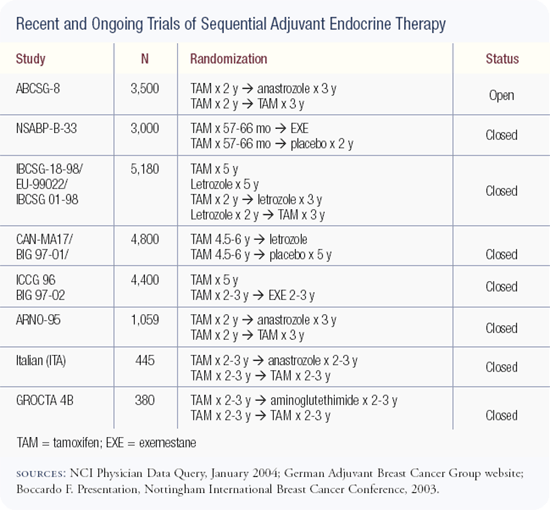
We completed accrual to an adjuvant trial (IBCSG-18-98) comparing five years of tamoxifen, five years of letrozole, two years of tamoxifen followed by three years of letrozole, and two years of letrozole followed by three years of tamoxifen in postmenopausal patients with endocrine-responsive disease. This trial accrued 8,028 patients.
A lifelong treatment strategy for patients with an increased risk of breast cancer recurrence might be reasonable. I think maintaining the cells under control and suppressing new tumors requires a sequential approach that includes endocrine therapy for tumors that are endocrine responsive.
— Aron Goldhirsch, MD
Aromatase inhibitors as initial therapy and sequence after tamoxifen
Increasingly, more data are emerging to support the superiority of aromatase inhibitors over tamoxifen. The NCIC-MA17 trial demonstrated the value of letrozole after five years of tamoxifen, and the Italian trial reported in San Antonio indicated that the switch from tamoxifen to anastrozole at two or three years results in a disease-free survival advantage and nearly results in a statistically significant survival advantage (p = 0.06).
I believe that if you're going to use an aromatase inhibitor, it is most appropriate to use it up front. The data in this setting are with anastrozole, so if I am going to use an aromatase inhibitor up front, I use anastrozole. The data for switching from tamoxifen at two to three years are with anastrozole, so I use anastrozole in that setting. After five years of tamoxifen, the data are with letrozole, so I use letrozole in those patients. Good clinical scientists treat patients according to the data.
— Anthony Howell, MD, MSc, FRCP
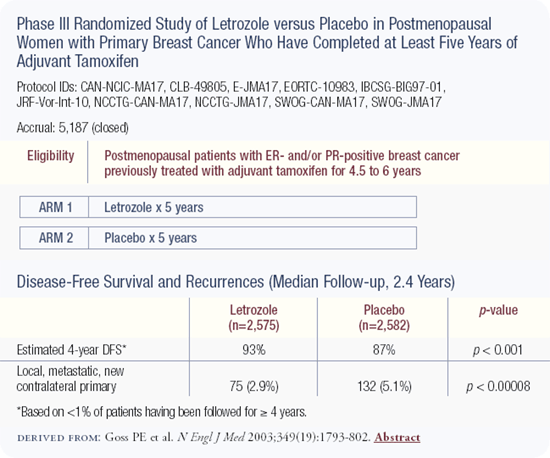
CAN-NCIC-MA17 trial: Efficacy of aromatase inhibitors following five of adjuvant tamoxifen
Led by the National Cancer Institute of Canada, MA17 randomly assigned over 5,000 postmenopausal women who had received tamoxifen for between four and a half and six years and were free of tumor, to receive letrozole or a placebo. Letrozole reduced the rate of breast cancer events by about 50 percent, including the risk of distant metastases and the risk of ipsilateral or contralateral breast cancer. The differences were so robust after only two and a half years that the study was closed before completing its planned five-year duration.
The data are exciting because letrozole has the potential to improve the long-term prognosis for the largest demographic group of patients — postmenopausal women with hormone receptor-positive breast cancer. Historically, these women have been offered five years of tamoxifen; now many such patients should consider taking letrozole after completing that therapy.
It's always exciting to close a study early because of such good news, but follow-up trials are needed to address unanswered questions about the best way to use letrozole in this setting. Also, there are concerns regarding the profound estrogen deprivation effects of aromatase inhibitors, particularly osteoporosis. We can study those issues, and potential interventions, but it means that we have to pause before blindly recommending this therapy to everyone.
— Harold J Burstein, MD, PhD
Time since completion of tamoxifen and aromatase inhibitor
MA17 was open to women who had finished tamoxifen within the past three months, but we have no data for women who have been off tamoxifen for a longer period. In practice, I consider letrozole therapy for patients who have finished their five years of tamoxifen therapy within the past year. Beyond year six, women who have had no recurrences have an additional period of time during which they've done well, and that means their moving-forward risk is even lower than it was before. It's difficult to know whether or not the data apply to them.
The whole issue of the timing, duration and sequencing of antiestrogen strategies is very interesting, and everyone is looking forward to the results of the Breast International Group/FemaraR-Tamoxifen (BIG/FEMTA) study. This large European trial has four arms: (1) five years of an aromatase inhibitor, (2) five years of tamoxifen, (3) two years of tamoxifen followed by three years of an aromatase inhibitor, and (4) two years of an aromatase inhibitor followed by three years of tamoxifen.
— Harold J Burstein, MD, PhD
A good clinical scientist and clinician will treat patients according to the available data. In the adjuvant setting, the ATAC data support using anastrozole up front, the Boccardo data support switching to anastrozole after two to three years of tamoxifen, and MA17 supports the use of letrozole after five years of tamoxifen.
So at this point, if you are starting adjuvant therapy, you should use anastrozole because we have data on that. If you are going to switch at two to three years, you switch to anastrozole because we have data on that. But if you're going to give treatment after five years, you use letrozole because we have data on that.
— Anthony Howell, MD, MSc, FRCP
In the ITA trial, patients received a total of five years of therapy — either tamoxifen alone or tamoxifen for at least two years followed by anastrozole. Results from the ITA trial confirm the data from the MA17 trial in which patients received five years of adjuvant tamoxifen and then an aromatase inhibitor. It is unknown whether 10 years of an adjuvant aromatase inhibitor alone would be more effective than five years of adjuvant tamoxifen followed by five years of an adjuvant aromatase inhibitor. Although the ITA trial was a small study, I'm willing to accept it as being fundamentally correct because the results are consistent with those from the MA17 trial. In both trials, a clear advantage was demonstrated for the crossover to an aromatase inhibitor after tamoxifen.
— I Craig Henderson, MD
Select publications
|
