|
You are here: Home: Special Report: 21st Annual Miami Breast Cancer Conference 2004:
H: Ductal Carcinoma In Situ

| RESEARCH LEADER COMMENTARY |
 |
Trends in the diagnosis of DCIS
In 1978, the American College of Surgeons conducted a survey demonstrating that 200 out of 24,000 cases of breast cancer were DCIS — less than one percent. The incidence of DCIS exploded in the mammographic era. By screening women, we discovered microcalcifications and other architectural distortions that we otherwise never would have known were present. Some of those women would have developed invasive breast cancer six to 10 years later. Now, we intercede in the neoplastic continuum five to 10 years earlier. Today, DCIS represents 21 percent of all new cancers. In 2003, we will detect 57,000 cases of DCIS and 211,000 cases of invasive breast cancer.
DCIS is the precursor lesion to invasive breast cancer. Roland Holland, the renowned Dutch pathologist, examined 100 consecutive invasive breast cancers, which he thoroughly sampled with multiple slides for each. In 98 out of 100 cases, he found a DCIS component in at least one of the slides. This is compelling evidence that DCIS is a precursor lesion. It does not mean all DCIS will develop into invasive breast cancer; rather, all invasive breast cancers were probably born from DCIS.
— Melvin Silverstein, MD
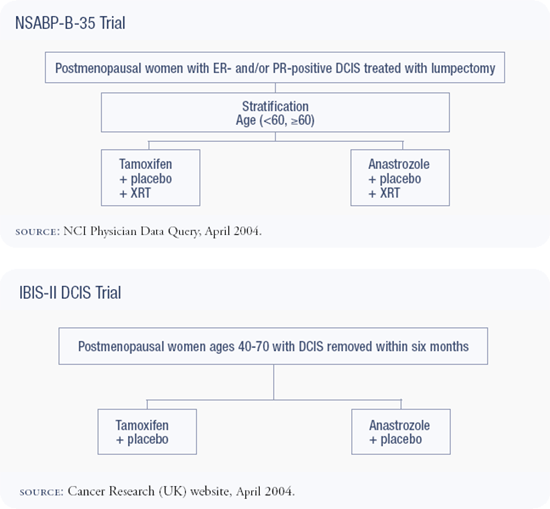
NSABP-B-35 trial : Anastrozole versus tamoxifen in DCIS
NSABP-B-35 is the next protocol in a generation of NSABP DCIS trials: B-17 compared radiotherapy to no treatment, B-24 added tamoxifen to lumpectomy and radiotherapy, and B-35, which opened in January 2003, compares anastrozole to tamoxifen for five years. We're hoping that anastrozole will be superior to tamoxifen, as it was in the ATAC trial; however, that trial was powered to detect small differences in efficacy.
We debated considerably whether ER positivity should be required for eligibility in B-35. Dr Craig Allred reanalyzed data from NSABP-B-24 and demonstrated benefit from tamoxifen only in patients with ER-positive DCIS. Ultimately, we decided to limit eligibility for B-35 to patients with ER-positive DCIS. Only a small subset of women with DCIS — approximately 20 percent — is ER-negative. At the current time, I believe it is overly restrictive and authoritarian to dictate that the community standard require estrogen receptor assay prior to treating DCIS.
— Norman Wolmark, MD
The NSABP study comparing tamoxifen and anastrozole for patients with DCIS is essentially a trial aimed at preventing invasive breast cancer. Aromatase inhibitors have emerged as very good agents in the treatment of metastatic breast cancer, both second- and first-line, and the pivotal results from the ATAC trial demonstrated adjuvant anastrozole was more effective than tamoxifen in reducing recurrence rates and contralateral breast cancers. If patients with DCIS fail, it's usually in the ipsilateral or contralateral breast rather than in the regional nodes or distant sites.
Aromatase inhibitors are very well-tolerated in general. In the ATAC trial, the safety profile of anastrozole was impressive. Patients had fewer thromboembolic events, endometrial cancers and menopausal symptoms than with tamoxifen, but with aromatase inhibitors we need to monitor bone density and fractures.
— Eleftherios P Mamounas, MD, MPH,
The question about aromatase inhibitors as preventive agents is a very important one. I am concerned that the IBIS-II trial — comparing anastrozole to placebo — won't give us the answer we need. We'll know if anastrozole is better than a placebo, but we won't know how SERMs compare to aromatase inhibitors or which is better in terms of overall health. We will not be able to extrapolate these answers from two completely different study populations, and this will leave us with another trial to do. In addition, I would not recommend this trial to a woman at very high risk. With tamoxifen on the market, proven to reduce breast cancer risk, I don't think taking a 50 percent chance of being randomized to a placebo is a good choice. IBIS-II also has a randomization for women with DCIS, but this compares anastrozole to tamoxifen.
I agree that treating DCIS is primarily prevention — it's a lesion that carries a significantly increased risk of invasive breast cancer. We tend to think of it differently because we treat it like cancer, but the question is the same. The NSABP-B-35 trial is asking the same question, randomly assigning women with DCIS to anastrozole versus tamoxifen. It is a good trial, addressing an important question, and I heartily support that study.
— Monica Morrow, MD
Select publications
|
|
