 |
| I N T E R V I E W |
 |
|
| Harry D Bear, MD, PhD |
| Dr Bear is Chairman of the Division of Surgical Oncology, Professor of Surgery and Microbiology and Immunology and Walter Lawrence Jr Distinguished Professor in Oncology at Virginia Commonwealth University School of Medicine’s Massey Cancer Center in Richmond, Virginia. |
 |
|
|
| Tracks 1-13 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
NSABP-B-27: Neoadjuvant AC
versus neoadjuvant AC followed
by docetaxel versus neoadjuvant
AC followed by adjuvant docetaxel |
| Track 3 |
AC followed by docetaxel as
adjuvant therapy |
| Track 4 |
NSABP-B-30: AC followed
by docetaxel versus TAC
versus AT as adjuvant therapy
for patients with nodepositive
disease |
| Track 5 |
Potential design of future NSABP
neoadjuvant/adjuvant trial |
| Track 6 |
Management of patients with
residual tumor burden
following surgery |
|
| Track 7 |
NSABP-B-35: Anastrozole versus
tamoxifen for DCIS |
| Track 8 |
NSABP-B-32 trial of sentinel
lymph node biopsy |
| Track 9 |
Development and validation of the
Oncotype DX™ assay |
| Track 10 |
NSABP-B-39: Conventional
whole breast irradiation versus
partial breast irradiation |
| Track 11 |
Potential benefits of core needle
biopsy versus excisional biopsy |
| Track 12 |
Selection of surgical
treatment following
neoadjuvant chemotherapy |
| Track 13 |
Use of ferromagnetic particles in
conjunction with MRI to identify
positive lymph nodes |
|
|
Select Excerpts from the Interview*
* Conducted on December 10, 2004
 Track 2 Track 2
 DR LOVE: Can you summarize the updated NSABP trial B-27 data? DR LOVE: Can you summarize the updated NSABP trial B-27 data? |
 DR BEAR: Although the numbers are low, the addition of docetaxel, preoperatively,
in NSABP-B-27 doubled the pathologic complete response rate from
13 percent to 26 percent. In 2004, we presented the overall and disease-free
survival data at San Antonio (Bear 2004). DR BEAR: Although the numbers are low, the addition of docetaxel, preoperatively,
in NSABP-B-27 doubled the pathologic complete response rate from
13 percent to 26 percent. In 2004, we presented the overall and disease-free
survival data at San Antonio (Bear 2004).
The overall survival did not show any significant difference among the groups
or between each of the docetaxel groups and AC alone. There was a trend
towards improved disease-free survival with the addition of docetaxel, particularly
when given preoperatively. When we examined relapse-free survival,
which was similar to the definition of disease-free survival in the CALGB trial 9344 (Henderson 2003), we saw a significant improvement with the addition
of preoperative docetaxel compared to AC alone.
The difference between the relapse-free survival result and the disease-free
survival result was probably caused by a chance event, which was an increase
in the number of second malignancies at other sites and in the contralateral
breast in some of the patients who received docetaxel. Since that’s included
in disease-free survival, it sort of wipes out the beneficial effect. Most of the
relapse-free survival benefit was caused by a reduction in local recurrences.
We have not yet seen any difference in distant disease-free survival, which, in
terms of patient survival, is probably the most important outcome.
 DR LOVE: What’s your interpretation of these data? DR LOVE: What’s your interpretation of these data?
 DR BEAR: I believe it relates to a number of factors, some of which are
biological. The Skipper hypothesis is that metastatic clones of tumor cells
behave differently from the primary tumor. We thought we had disproven that
in the NSABP-B-18 trial (Fisher 1998), but it may be true in some patients, so
that a patient who has a good response in the breast may not necessarily have a
good response in the metastatic disease sites. DR BEAR: I believe it relates to a number of factors, some of which are
biological. The Skipper hypothesis is that metastatic clones of tumor cells
behave differently from the primary tumor. We thought we had disproven that
in the NSABP-B-18 trial (Fisher 1998), but it may be true in some patients, so
that a patient who has a good response in the breast may not necessarily have a
good response in the metastatic disease sites.
Probably the most important reason is a statistical issue.
One of the other major factors is that this group of patients, as compared to
the CALGB-9344 (Henderson 2003) or NSABP-B-28 (Mamounas 2005)
trials, is probably diluted by some better-risk patients. In those trials, all the
patients had known positive nodes, whereas in B-27, the patients’ nodal status
is unknown and certainly included a number of ER-positive, node-negative
patients whose benefit from any chemotherapy is probably fairly small.
If you look at the actual magnitude of the effect in terms of hazard ratios, it’s
really quite similar to CALGB-9344 or NSABP-B-28 with the addition of
paclitaxel, so I think it’s largely a sample size issue, as well as some potential
biostatistical parameters that neutralized any effect on survival.
 Track 7 Track 7
 DR LOVE: What’s going on with NSABP-B-35, the trial in DCIS? DR LOVE: What’s going on with NSABP-B-35, the trial in DCIS? |
 DR BEAR: That is a very interesting trial, and it’s continuing to accrue very
well. That study is examining what kind of an anti-estrogen should be given
to patients with ER-positive DCIS. All the patients have been treated for
DCIS with a lumpectomy and radiation therapy, and they are then randomly
assigned to tamoxifen or anastrozole. DR BEAR: That is a very interesting trial, and it’s continuing to accrue very
well. That study is examining what kind of an anti-estrogen should be given
to patients with ER-positive DCIS. All the patients have been treated for
DCIS with a lumpectomy and radiation therapy, and they are then randomly
assigned to tamoxifen or anastrozole.
 DR LOVE: What’s been your experience in terms of tolerance of the aromatase
inhibitors and anastrozole, as compared to tamoxifen? DR LOVE: What’s been your experience in terms of tolerance of the aromatase
inhibitors and anastrozole, as compared to tamoxifen?
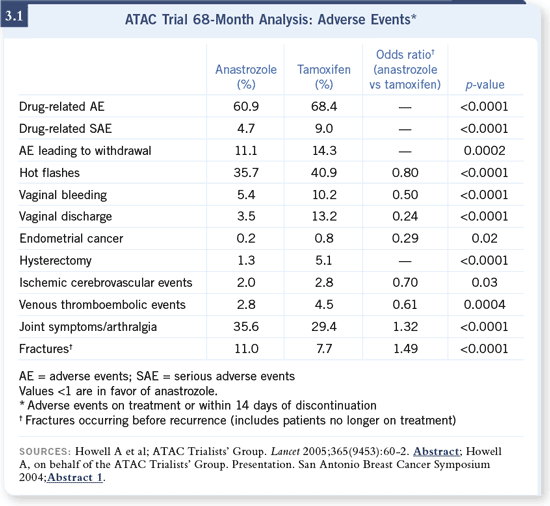
 DR BEAR: For the most part, they’re very well tolerated. Patients seem to do
quite well with anastrozole, and I think it’s somewhat less of a problem with
hot f lashes and other side effects, compared to tamoxifen (3.1). However,
aromatase inhibitors are certainly not trouble free. DR BEAR: For the most part, they’re very well tolerated. Patients seem to do
quite well with anastrozole, and I think it’s somewhat less of a problem with
hot f lashes and other side effects, compared to tamoxifen (3.1). However,
aromatase inhibitors are certainly not trouble free.
 DR LOVE: What’s your take on the ATAC data (Howell 2005) and some of
the other data that have been coming out in terms of switching aromatase
inhibitors (Boccardo 2005; Coombes 2004; Goss 2003; Jakesz 2005a, b)? DR LOVE: What’s your take on the ATAC data (Howell 2005) and some of
the other data that have been coming out in terms of switching aromatase
inhibitors (Boccardo 2005; Coombes 2004; Goss 2003; Jakesz 2005a, b)?
 DR BEAR: I believe it’ll probably precipitate a fairly wholesale change in the
primary treatment of ER-positive postmenopausal breast cancer patients. It
clearly is the end of five years of tamoxifen as a standard treatment; I don’t
think we’ll see that anymore. DR BEAR: I believe it’ll probably precipitate a fairly wholesale change in the
primary treatment of ER-positive postmenopausal breast cancer patients. It
clearly is the end of five years of tamoxifen as a standard treatment; I don’t
think we’ll see that anymore.
 Track 8 Track 8
 DR LOVE: What’s the status of the NSABP sentinel lymph node trial
— B-32? DR LOVE: What’s the status of the NSABP sentinel lymph node trial
— B-32? |
 DR BEAR: Dr Julian presented the initial results from that trial at San Antonio
in 2004, which consisted of the pathologic data and accuracy data for the half
of the trial that received a sentinel node biopsy plus an axillary node dissection
( Julian 2004). This was a trial of 5,600 patients randomly assigned to sentinel
node biopsy with or without an axillary node dissection. DR BEAR: Dr Julian presented the initial results from that trial at San Antonio
in 2004, which consisted of the pathologic data and accuracy data for the half
of the trial that received a sentinel node biopsy plus an axillary node dissection
( Julian 2004). This was a trial of 5,600 patients randomly assigned to sentinel
node biopsy with or without an axillary node dissection.
The false-negative rate in that trial was 9.5 percent, which some people
thought was higher than expected, but it’s right in line with other multicenter
validation trials. There are individual institutional trials with much
lower rates done by a few surgeons. Immunohistochemistry examination of
the sentinel nodes was done as part of the trial, but it was done centrally, and
it was blinded, so we don’t know the results of those.
 DR LOVE: What is the current role of sentinel node biopsy in the
clinical setting? DR LOVE: What is the current role of sentinel node biopsy in the
clinical setting?
 DR BEAR: I think it probably should be the standard of care for most patients
who don’t have a specific contraindication to it. It has been in my practice
since the completion of the trial. That’s what I offer most patients with
invasive breast cancer. DR BEAR: I think it probably should be the standard of care for most patients
who don’t have a specific contraindication to it. It has been in my practice
since the completion of the trial. That’s what I offer most patients with
invasive breast cancer.
 DR LOVE: Does the false-negative rate of 9.5 percent concern you? DR LOVE: Does the false-negative rate of 9.5 percent concern you?
 DR BEAR: It doesn’t really concern me. I feel pretty confident in the
technique. We’ve been doing it a long time, and I’ve seen very few, if any,
axillary recurrences that were unexpected, and that’s probably the most
significant thing that could happen. Obviously, the main answer to that
question really is going to have to come from the long-term follow-up of the
B-32 trial. DR BEAR: It doesn’t really concern me. I feel pretty confident in the
technique. We’ve been doing it a long time, and I’ve seen very few, if any,
axillary recurrences that were unexpected, and that’s probably the most
significant thing that could happen. Obviously, the main answer to that
question really is going to have to come from the long-term follow-up of the
B-32 trial.
 Track 9 Track 9
 DR LOVE: Can you discuss the development and validation of the
Oncotype DX assay? DR LOVE: Can you discuss the development and validation of the
Oncotype DX assay? |
 DR BEAR: This is an exciting era in the management of breast cancer.
Soonmyung Paik and the NSABP have developed Oncotype DX, a genomic
assay that reliably evaluates gene expression in paraffin-fixed tumor tissue.
They obtained tumor tissue from a large number of the patients participating
in the NSABP-B-14 trial and, using the assay, established a panel of 21 genes
that were accurate at predicting the risk of recurrence among patients who
were randomly assigned to either tamoxifen or placebo (Paik 2004a). DR BEAR: This is an exciting era in the management of breast cancer.
Soonmyung Paik and the NSABP have developed Oncotype DX, a genomic
assay that reliably evaluates gene expression in paraffin-fixed tumor tissue.
They obtained tumor tissue from a large number of the patients participating
in the NSABP-B-14 trial and, using the assay, established a panel of 21 genes
that were accurate at predicting the risk of recurrence among patients who
were randomly assigned to either tamoxifen or placebo (Paik 2004a).
The panel of 21 genes as a predictor of recurrence — called the risk score —
was validated in the NSABP-B-20 trial. In B-20, patients with node-negative,
ER-positive disease were randomly assigned to tamoxifen alone or tamoxifen
plus chemotherapy. In that group of patients, not only did the risk score
estimate the risk of recurrence, it also significantly predicted whether or not
patients would receive any benefit from adding chemotherapy to tamoxifen.
Only the patients in the high-risk group — not the low- or intermediate-risk
group — showed significant benefit from adding chemotherapy to tamoxifen
(Paik 2004b).
A lot of unanswered questions remain, including patients with node-positive
disease who are already at higher risk, a priori. Additionally, does this assay apply to other types of patients? That is one of the things we’re going to assess
in the neoadjuvant setting in the NSABP-B-40 study.
 DR LOVE: Are the patients in the NSABP-B-40 neoadjuvant trial going to be
evaluated with a genomic assay? DR LOVE: Are the patients in the NSABP-B-40 neoadjuvant trial going to be
evaluated with a genomic assay?
 DR BEAR: In the B-40 trial, core biopsies will be taken and retrieved for the
purpose of gene expression analysis. We will be able to look at that panel of 21
genes as one of the ways to assess gene expression, which is a critical part of
the study. DR BEAR: In the B-40 trial, core biopsies will be taken and retrieved for the
purpose of gene expression analysis. We will be able to look at that panel of 21
genes as one of the ways to assess gene expression, which is a critical part of
the study.
SELECT PUBLICATIONS
|

