 |
| I N T E R V I E W |
 |
|
| Rowan T Chlebowski, MD, PhD |
| Dr Chlebowski is a Professor of Medicine at UCLA School of Medicine and Chief of Medical Oncology at Harbor-UCLA Medical Center in Torrance, California. |
 |
|
|
| Tracks 1-16 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
Women’s Intervention Nutrition
Study: Dietary fat reduction in
postmenopausal women with
primary breast cancer |
| Track 3 |
Impact of dietary fat reduction on
recurrence according to
ER phenotype |
| Track 4 |
Results from Women’s Health
Initiative trials of postmenopausal
hormone therapy |
| Track 5 |
Tolerability of aromatase inhibitors
versus tamoxifen |
| Track 6 |
Increased incidence of cardiac
toxicity and stroke associated with
letrozole in BIG 1-98 |
| Track 7 |
Mathematical modeling to
determine optimal adjuvant
hormonal therapy |
| Track 8 |
Role of bisphosphonates to offset
aromatase inhibitor-induced
bone loss |
|
| Track 9 |
Selection of up-front
hormonal therapy |
| Track 10 |
Clinical use of ovarian ablation
with an aromatase inhibitor |
| Track 11 |
Time course for switching
from tamoxifen to an
aromatase inhibitor |
| Track 12 |
Management of postmenopausal
women after five years of
adjuvant tamoxifen |
| Track 13 |
Rationale for up-front use of
aromatase inhibitors |
| Track 14 |
Postmenopausal hormone
therapy for breast
cancer survivors |
| Track 15 |
Potential role of tibolone to
alleviate menopausal symptoms
in patients with breast cancer |
| Track 16 |
Impact of race and ethnicity on
breast cancer histology subtype |
|
|
Select Excerpts from the Interview*
* Conducted on May 20, 2005
 Tracks 2-3 Tracks 2-3
 DR LOVE: Can you discuss the Women’s Intervention Nutrition Study
(WINS) that you presented at ASCO? DR LOVE: Can you discuss the Women’s Intervention Nutrition Study
(WINS) that you presented at ASCO? |
 DR CHLEBOWSKI: The issue of dietary fat intake and breast cancer has been
around for about 25 years. So to address this issue, we conducted a randomized clinical trial. We entered 2,437 women aged 48 to 79 from 37 clinical
centers in the United States. They all received standard breast cancer management,
including surgery, radiation therapy if indicated, tamoxifen for five years
if estrogen receptor-positive and a defined chemotherapy if estrogen receptornegative.
The estrogen receptor-positive patients could also receive chemotherapy. DR CHLEBOWSKI: The issue of dietary fat intake and breast cancer has been
around for about 25 years. So to address this issue, we conducted a randomized clinical trial. We entered 2,437 women aged 48 to 79 from 37 clinical
centers in the United States. They all received standard breast cancer management,
including surgery, radiation therapy if indicated, tamoxifen for five years
if estrogen receptor-positive and a defined chemotherapy if estrogen receptornegative.
The estrogen receptor-positive patients could also receive chemotherapy.
 DR LOVE: What was the rationale for looking at nutrition and breast cancer
recurrence? DR LOVE: What was the rationale for looking at nutrition and breast cancer
recurrence?
 DR CHLEBOWSKI: The whole issue probably began with the country-to-country
differences in breast cancer incidence. Twenty-five years ago, the
Japanese not only had fewer cancers but they had a much lower recurrence
rate, and at that time, the difference in obesity between American and
Japanese women wasn’t that great. DR CHLEBOWSKI: The whole issue probably began with the country-to-country
differences in breast cancer incidence. Twenty-five years ago, the
Japanese not only had fewer cancers but they had a much lower recurrence
rate, and at that time, the difference in obesity between American and
Japanese women wasn’t that great.
There were a number of studies that looked at cohorts and suggested that
dietary fat intake would affect recurrence. More recently, the attention in
the observational studies has shifted away from dietary fat and more towards
obesity and physical activity.
 DR LOVE: When you look at those components — dietary fat, obesity and
physical activity — what would be the mechanism of action as to why it
would affect breast cancer recurrence? DR LOVE: When you look at those components — dietary fat, obesity and
physical activity — what would be the mechanism of action as to why it
would affect breast cancer recurrence?
 DR CHLEBOWSKI: The prevailing thought was that obesity or dietary fat
could be related to estrogen levels, and they can show that association. Of
course, that would make it much less interesting if that were the only mechanism
because now we have aromatase inhibitors. DR CHLEBOWSKI: The prevailing thought was that obesity or dietary fat
could be related to estrogen levels, and they can show that association. Of
course, that would make it much less interesting if that were the only mechanism
because now we have aromatase inhibitors.
One always had the consideration that it is more like the metabolic syndrome
where you end up having obesity, insulin resistance associated with coronary
heart disease, dementia, diabetes and the cancers — colon and breast cancer
— at least in some of those studies. That kind of mechanism, metabolic
syndrome, insulin regulatory pathways, had also been under consideration but
not the primary focus.
 DR LOVE: What were the endpoints of the study? DR LOVE: What were the endpoints of the study?
 DR CHLEBOWSKI: Our primary study endpoint was relapse-free survival,
which included all breast cancer recurrence sites, including contralateral breast
cancers. We found that the dietary group had a longer relapse-free survival
than the control population. 12.4 percent of the control group had a relapse
compared to 9.8 in the diet group, which was a 2.6 percent absolute difference
at five years or a 24 percent reduction in risk of recurrence. DR CHLEBOWSKI: Our primary study endpoint was relapse-free survival,
which included all breast cancer recurrence sites, including contralateral breast
cancers. We found that the dietary group had a longer relapse-free survival
than the control population. 12.4 percent of the control group had a relapse
compared to 9.8 in the diet group, which was a 2.6 percent absolute difference
at five years or a 24 percent reduction in risk of recurrence.
 DR LOVE: Do you think this is now something that should be brought up to
women with breast cancer? DR LOVE: Do you think this is now something that should be brought up to
women with breast cancer?
 DR CHLEBOWSKI: We’re not quite there yet in that we recognize the need
for further follow-up, a peer-review publication and probably a confirmatory
study. Having said that, the diet was associated with nutritional adequacy and can be recommended for other health reasons and would also have no appreciable
side effects. DR CHLEBOWSKI: We’re not quite there yet in that we recognize the need
for further follow-up, a peer-review publication and probably a confirmatory
study. Having said that, the diet was associated with nutritional adequacy and can be recommended for other health reasons and would also have no appreciable
side effects.
The only issue that I could see is this guilt factor. For instance, the American
Cancer Society has recommendations for dietary change after cancer diagnosis.
They’re all based on inference. Now we have a little signal that there might
be a cancer benefit as well, but I don’t think we would tell every patient that
they have to do it. If somebody wanted to do something, you’d say, “Here’s
something that you could do. It may inf luence your breast cancer, but it also
has other potential benefits.”
 Track 4 Track 4
 DR LOVE: Can you update us on the Women’s Health Initiative (WHI)
trials and summarize some of the most important findings that have
come out in the last couple of years, particularly related to breast cancer? DR LOVE: Can you update us on the Women’s Health Initiative (WHI)
trials and summarize some of the most important findings that have
come out in the last couple of years, particularly related to breast cancer? |
 DR CHLEBOWSKI: There are two key areas. First would be the WHI
hormone trials, and the initial data reported was the comparison of estrogen
plus progestin versus placebo in women who had a uterus (Rossouw 2002).
Basically, the surprising finding was that coronary heart disease was increased
by approximately 25 to 30 percent, as opposed to the prestudy estimates
that anticipated it would be reduced with hormone use. Breast cancers were
increased, as expected, but surprisingly, prognostic characteristics worsened.
After one year of estrogen plus progestin use, abnormal mammograms
increased by 74 percent. DR CHLEBOWSKI: There are two key areas. First would be the WHI
hormone trials, and the initial data reported was the comparison of estrogen
plus progestin versus placebo in women who had a uterus (Rossouw 2002).
Basically, the surprising finding was that coronary heart disease was increased
by approximately 25 to 30 percent, as opposed to the prestudy estimates
that anticipated it would be reduced with hormone use. Breast cancers were
increased, as expected, but surprisingly, prognostic characteristics worsened.
After one year of estrogen plus progestin use, abnormal mammograms
increased by 74 percent.
These were some of the major findings. Basically, what happened with that
was the FDA changed the labeling of estrogen plus progestin, and 33 million
fewer prescriptions for menopausal hormone therapy were written in 2003
versus 2001.
Also, the trial of estrogen only versus placebo for otherwise healthy
women who had a prior hysterectomy was stopped early. In that study, there
was no effect on the global health index — the overall balance of risks and
benefits (Anderson 2004). Actually, coronary heart disease didn’t increase.
Rather, there was approximately a nine percent decrease, but it was not statistically
significant.
The other very interesting finding was that estrogen-only therapy ended up
trending towards a decrease in breast cancers. There were approximately 24
percent fewer breast cancers on the estrogen-only arm. We’re in the process
of doing further analyses, and I believe this may well represent a real event.
Short-term estrogen may well be associated with a reduction in breast
cancer risk.
 Track 5 Track 5
 DR LOVE: Can you summarize where we are in terms of the major
randomized trials of aromatase inhibitors? DR LOVE: Can you summarize where we are in terms of the major
randomized trials of aromatase inhibitors? |
 DR CHLEBOWSKI: We have 68 months’ follow-up on the ATAC data, which
means the majority of patients have completed their therapy and approximately
a year of follow-up afterwards (Howell 2005). I see the toxicity data as being
final, unless one proposes a new mechanism whereby returning estrogen back
to the physiological levels causes different toxicities. The five-year toxicity
data is very favorable for anastrozole compared to tamoxifen, because the three
life-threatening toxicities — endometrial cancer, arterial and venous vascular
events — were all significantly less with anastrozole. DR CHLEBOWSKI: We have 68 months’ follow-up on the ATAC data, which
means the majority of patients have completed their therapy and approximately
a year of follow-up afterwards (Howell 2005). I see the toxicity data as being
final, unless one proposes a new mechanism whereby returning estrogen back
to the physiological levels causes different toxicities. The five-year toxicity
data is very favorable for anastrozole compared to tamoxifen, because the three
life-threatening toxicities — endometrial cancer, arterial and venous vascular
events — were all significantly less with anastrozole.
 DR LOVE: While endometrial cancer is certainly a disturbing event, I guess
you could raise the question as to whether it is really life threatening. DR LOVE: While endometrial cancer is certainly a disturbing event, I guess
you could raise the question as to whether it is really life threatening.
 DR CHLEBOWSKI: Endometrial cancer has a 15 percent mortality rate associated
with it. When we look at the hip fractures, which are life threatening,
the incidence was low and really not different at all between the patients on
anastrozole versus tamoxifen. Tony Howell just reported on the cardiac deaths
data, which were 49 versus 46 — really no difference at all after five years. DR CHLEBOWSKI: Endometrial cancer has a 15 percent mortality rate associated
with it. When we look at the hip fractures, which are life threatening,
the incidence was low and really not different at all between the patients on
anastrozole versus tamoxifen. Tony Howell just reported on the cardiac deaths
data, which were 49 versus 46 — really no difference at all after five years.
 Track 13 Track 13
 DR LOVE: Putting aside toxicities, there has been a lot of discussion
about whether the long-term — 10-, 15-, 20-year — relapse rate in
some patients would be lower starting with tamoxifen for some period
of time, followed by an aromatase inhibitor. What are your thoughts on
that hypothesis? DR LOVE: Putting aside toxicities, there has been a lot of discussion
about whether the long-term — 10-, 15-, 20-year — relapse rate in
some patients would be lower starting with tamoxifen for some period
of time, followed by an aromatase inhibitor. What are your thoughts on
that hypothesis? |
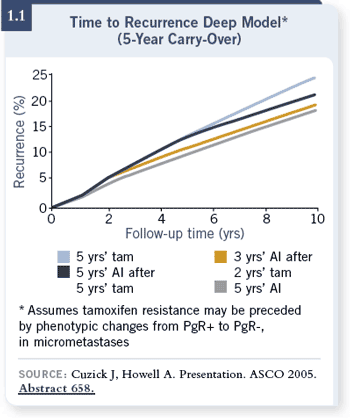
 DR CHLEBOWSKI: If you start with tamoxifen, after two and a half, three or
five years, more patients will have relapsed than on an aromatase inhibitor
(1.1). A substantial number of those patients will be irretrievable — they have
incurable disease — and so you’re banking on the fact that you’ll be able to
capture more patients later, but we don’t have any data for that. That’s
just speculation. DR CHLEBOWSKI: If you start with tamoxifen, after two and a half, three or
five years, more patients will have relapsed than on an aromatase inhibitor
(1.1). A substantial number of those patients will be irretrievable — they have
incurable disease — and so you’re banking on the fact that you’ll be able to
capture more patients later, but we don’t have any data for that. That’s
just speculation.
While I believe sequencing therapy may be better, ultimately, I still don’t see
any reason not to start with the most effective therapy. An aromatase inhibitor
followed by tamoxifen or a nonsteroidal aromatase inhibitor makes more sense
to me. We have to wait to see the data from the BIG FEMTA trial, which
includes an arm with letrozole as initial treatment followed by tamoxifen.
 Track 16 Track 16
 DR LOVE: Can you discuss the issue of race, ethnicity and breast
cancer subtypes? DR LOVE: Can you discuss the issue of race, ethnicity and breast
cancer subtypes? |
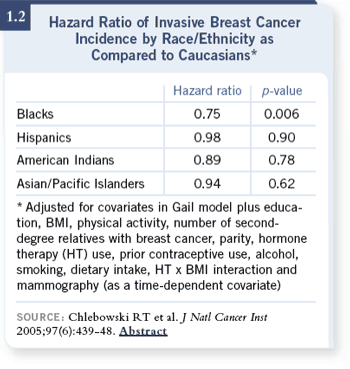
 DR CHLEBOWSKI: In the
Women’s Health Initiative,
we have a large population,
so we examined risk factors
for breast cancer by ethnicity
(Chlebowski 2005). We took
160,000 women, in whom we
have a racial ethnic distribution,
and tracked them
as a prospective cohort. As
expected, we found the ageadjusted
breast cancer risk for
African-Americans, Hispanics
and Asian Pacific Islanders,
compared to Caucasians, were
all substantially less — 30
percent or so less (1.2), which
is about the same as the
SEER data. DR CHLEBOWSKI: In the
Women’s Health Initiative,
we have a large population,
so we examined risk factors
for breast cancer by ethnicity
(Chlebowski 2005). We took
160,000 women, in whom we
have a racial ethnic distribution,
and tracked them
as a prospective cohort. As
expected, we found the ageadjusted
breast cancer risk for
African-Americans, Hispanics
and Asian Pacific Islanders,
compared to Caucasians, were
all substantially less — 30
percent or so less (1.2), which
is about the same as the
SEER data.
We corrected for the Gail
risk model, and their hazard
ratios moved towards unity
but were still different. We
looked at approximately 22
risk factors, so we really have
extensive risk-factor correction,
and what we found was
very interesting. We could
almost completely explain
the reduced risk of breast
cancer in Hispanics and
Asian Pacific Islanders. Their
hazard ratios, with our risk
factors in the final models,
were 0.98 and 0.94.
 DR LOVE: What about the
African-American population? DR LOVE: What about the
African-American population?
 DR CHLEBOWSKI: Interestingly,
for African-Americans, their hazard ratio compared to Caucasians was
0.75, which statistically was significantly lower. Even more interestingly, they
had substantially lower risk for hormone receptor-positive cancers — about 50
percent of the Caucasians’ risk. DR CHLEBOWSKI: Interestingly,
for African-Americans, their hazard ratio compared to Caucasians was
0.75, which statistically was significantly lower. Even more interestingly, they
had substantially lower risk for hormone receptor-positive cancers — about 50
percent of the Caucasians’ risk.
The most interesting finding was our equivalent of triple-negative cancers
— ER-negative, PR-negative and high grade. African-Americans have nearly a fivefold-increased risk of having triple-negative cancers compared to Caucasians.
In African-Americans, 31 percent of the cancers were triple negatives
compared to 10 percent in Caucasians.
Other groups are looking at whether the triple negatives have genetic profiles
similar to basaloid cancers, which are faster growing and more difficult to
treat. We consider this kind of a unifying hypothesis in that, with these
more common basaloid cancers, with the same mammographic screening
or frequency, you’re going to find higher-stage cancers, and with the same
therapy that was given in the cooperative groups, they will have a
worse outcome.
It’s interesting that in several African-American populations, the triple
negatives are close to 50 or 60 percent of the population, instead of a third.
Studies are now underway looking at the genetic profiles of these mixed
African-American populations compared to Caucasian populations.
SELECT PUBLICATIONS
|

