 |
| I N T E R V I E W |
 |
|
| Eric P Winer, MD |
| Dr Winer is the Director of the Breast Oncology Center at the Dana-Farber Cancer Institute and Associate Professor of Medicine at Harvard Medical School in Boston, Massachusetts. |
 |
|
|
| Tracks 1-17 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
ECOG-E2100: Paclitaxel with or
without bevacizumab as
first-line therapy for metastatic
breast cancer |
| Track 3 |
Clinical use of bevacizumab in
combination with chemotherapy |
| Track 4 |
Potential benefits of nanoparticle
albumin-bound (nab) paclitaxel |
| Track 5 |
Eligibility criteria for ECOG-E2100 |
| Track 6 |
Bevacizumab in the second- and
third-line settings |
| Track 7 |
Proposed ECOG pilot trial
examining the role of
adjuvant bevacizumab |
| Track 8 |
Combined analysis of
NSABP-B-31/NCCTG-N9831
trials of adjuvant trastuzumab |
| Track 9 |
Cardiac toxicity induced by
adjuvant trastuzumab |
|
| Track 10 |
Concurrent versus sequential
administration of adjuvant
trastuzumab and chemotherapy |
| Track 11 |
Adjuvant trastuzumab for patients
with node-negative disease |
| Track 12 |
Combining hormonal therapy with
trastuzumab and chemotherapy
in the adjuvant setting |
| Track 13 |
Time course for initiating therapy
with adjuvant trastuzumab |
| Track 14 |
Need to facilitate completion of
clinical trials more rapidly |
| Track 15 |
Future directions in adjuvant
trials for patients with HER2-
positive disease |
| Track 16 |
Management of patients who
relapse on adjuvant trastuzumab |
| Track 17 |
Monitoring cardiac function
in patients receiving
adjuvant trastuzumab |
|
|
Select Excerpts from the Interview*
* Conducted on June 25, 2005
 Tracks 3-4 Tracks 3-4
 DR LOVE: Cost and reimbursement issues aside, what are the clinical
implications of the ECOG-E2100 bevacizumab data to clinical practice? DR LOVE: Cost and reimbursement issues aside, what are the clinical
implications of the ECOG-E2100 bevacizumab data to clinical practice? |
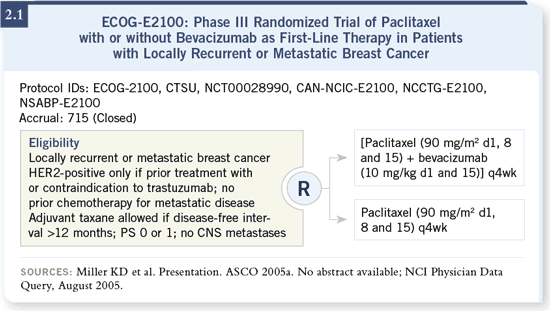
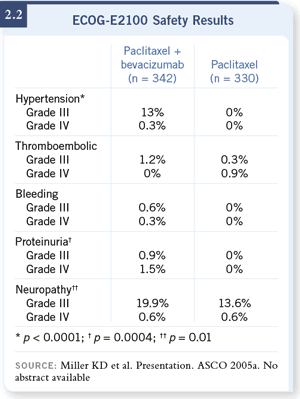
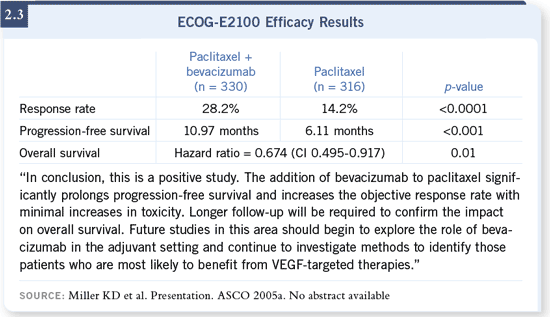
 DR WINER: I believe the results of ECOG-E2100 are impressive enough that,
in the absence of a contraindication to bevacizumab, I would now use it in a
first-line setting, optimally in combination with paclitaxel as administrated in
the study (Miller 2005a; [2.1, 2.2, 2.3]). DR WINER: I believe the results of ECOG-E2100 are impressive enough that,
in the absence of a contraindication to bevacizumab, I would now use it in a
first-line setting, optimally in combination with paclitaxel as administrated in
the study (Miller 2005a; [2.1, 2.2, 2.3]).
 I doubt that the interaction
is specific between paclitaxel
and bevacizumab, although
I’m well aware that when
given with capecitabine
in more advanced disease,
bevacizumab seemed to be
less active. I believe that’s
probably related to the
setting rather than the drug. I doubt that the interaction
is specific between paclitaxel
and bevacizumab, although
I’m well aware that when
given with capecitabine
in more advanced disease,
bevacizumab seemed to be
less active. I believe that’s
probably related to the
setting rather than the drug.
 DR LOVE: Of course, we
have seen bevacizumab
work with multiple different
agents, particularly in
colorectal cancer, and we’ve
seen less activity in the
second-line setting than
the first-line setting in
colorectal cancer. DR LOVE: Of course, we
have seen bevacizumab
work with multiple different
agents, particularly in
colorectal cancer, and we’ve
seen less activity in the
second-line setting than
the first-line setting in
colorectal cancer.
 DR WINER: I agree, and it’s
one of the reasons why
I tend to think this is
probably more the setting
than the drug. DR WINER: I agree, and it’s
one of the reasons why
I tend to think this is
probably more the setting
than the drug.
 DR LOVE: What about docetaxel versus paclitaxel, with bevacizumab? DR LOVE: What about docetaxel versus paclitaxel, with bevacizumab?
 DR WINER: I believe the two taxanes are more similar than they are different,
but we have data with paclitaxel and, specifically, we have data with a weekly
or almost weekly regimen. In the ECOG trial, paclitaxel was given three out
of four weeks. DR WINER: I believe the two taxanes are more similar than they are different,
but we have data with paclitaxel and, specifically, we have data with a weekly
or almost weekly regimen. In the ECOG trial, paclitaxel was given three out
of four weeks.
 DR LOVE: What are your thoughts about nanoparticle albumin-bound (nab)
paclitaxel with bevacizumab at this time and in the future? DR LOVE: What are your thoughts about nanoparticle albumin-bound (nab)
paclitaxel with bevacizumab at this time and in the future?
 DR WINER: At the moment, I wouldn’t be in a rush to give it with bevacizumab.
Once we have a little bit of data in terms of the safety of nab paclitaxel
with bevacizumab, I believe it would be a reasonable substitution in a limited
number of patients, such as a woman with a contraindication to paclitaxel
based on hypersensitivity. DR WINER: At the moment, I wouldn’t be in a rush to give it with bevacizumab.
Once we have a little bit of data in terms of the safety of nab paclitaxel
with bevacizumab, I believe it would be a reasonable substitution in a limited
number of patients, such as a woman with a contraindication to paclitaxel
based on hypersensitivity.
In the CALGB, we actually have planned a study comparing different schedules
of nab paclitaxel and paclitaxel. In that study, bevacizumab will be
combined with either paclitaxel or nab paclitaxel for those patients who don’t
have a contraindication to it.
 DR LOVE: Are you currently using nab paclitaxel in your practice? DR LOVE: Are you currently using nab paclitaxel in your practice?
 DR WINER: At our center, we elected to use it mostly, if not exclusively, in
patients who have either had hypersensitivity reactions to paclitaxel that we
thought were related to the cremophor or in patients who have trouble tolerating
steroid premedication. DR WINER: At our center, we elected to use it mostly, if not exclusively, in
patients who have either had hypersensitivity reactions to paclitaxel that we
thought were related to the cremophor or in patients who have trouble tolerating
steroid premedication.
 DR LOVE: Another issue with nab paclitaxel is the shorter infusion time. How
much of a benefit is this? DR LOVE: Another issue with nab paclitaxel is the shorter infusion time. How
much of a benefit is this?
 DR WINER: The shorter infusion time potentially has two benefits. One is
that it’s better for patients to spend less time in the clinic. We don’t want to
take over people’s lives. The second benefit is that, whether it’s in an academic
center or in practice, many medical oncologists and oncology nurses are struggling
with infusion rooms that are bursting at the seams because of all of these
new therapies, so minimizing the time a patient spends in the infusion room is
ultimately quite important. DR WINER: The shorter infusion time potentially has two benefits. One is
that it’s better for patients to spend less time in the clinic. We don’t want to
take over people’s lives. The second benefit is that, whether it’s in an academic
center or in practice, many medical oncologists and oncology nurses are struggling
with infusion rooms that are bursting at the seams because of all of these
new therapies, so minimizing the time a patient spends in the infusion room is
ultimately quite important.
In addition, I believe avoiding steroid premedication is a big deal. Steroids are
fine for some people the first or second time you take them, but after a while,
the ongoing ups and downs of steroids can be a real problem.
 Tracks 8-9 Tracks 8-9
 DR LOVE: Can you provide an overview of the adjuvant trastuzumab
data presented at ASCO? DR LOVE: Can you provide an overview of the adjuvant trastuzumab
data presented at ASCO? |
 DR WINER: The adjuvant trastuzumab data were pretty impressive and very
striking for all of us listening to the presentations (Romond 2005; Perez
2005b; Piccart-Gebhart 2005). I believe what made them that much more
striking is that the benefits were seen so early on, although if one thinks about
it, given the fact that events in patients with HER2-positive breast cancer tend
to be seen early on, maybe that’s not so surprising. DR WINER: The adjuvant trastuzumab data were pretty impressive and very
striking for all of us listening to the presentations (Romond 2005; Perez
2005b; Piccart-Gebhart 2005). I believe what made them that much more
striking is that the benefits were seen so early on, although if one thinks about
it, given the fact that events in patients with HER2-positive breast cancer tend
to be seen early on, maybe that’s not so surprising.
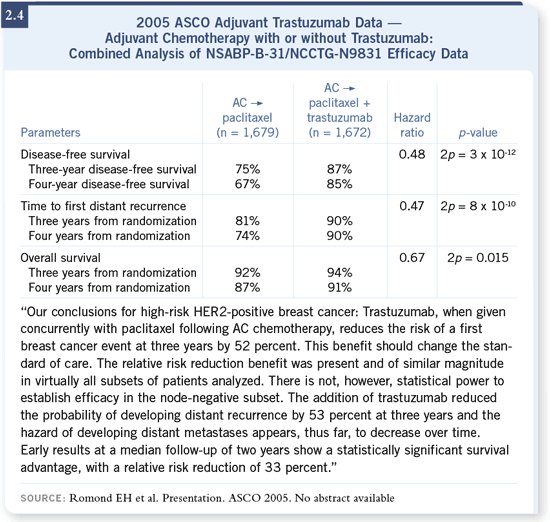
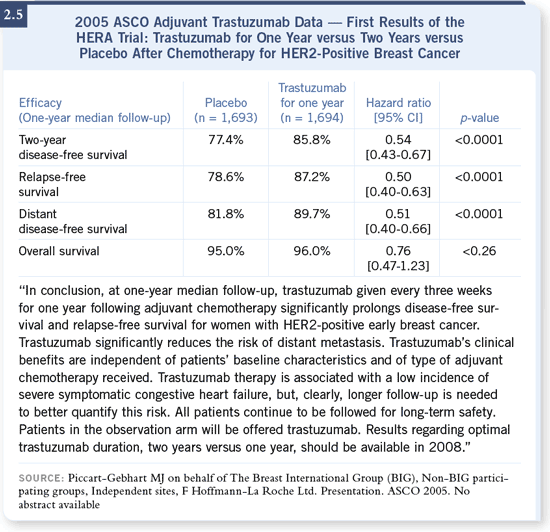
Essentially, there were results from one large study and another analysis of
two studies that were combined. The two US studies, which were analyzed
together, were the NSABP trial B-31 and the NCCTG Intergroup N9831
trial (Romond 2005; [2.4]). They analyzed the patients on the NSABP trial
who were randomly assigned to AC followed by paclitaxel versus those who
received AC followed by paclitaxel plus trastuzumab.
The NCCTG trial was more complicated and had a third arm in which
patients received sequential trastuzumab. That arm was not included in
the combined analysis, so essentially, the combined analysis compared AC
followed by paclitaxel versus AC followed by paclitaxel with trastuzumab,
followed by trastuzumab for a total of one year. The other trial was the
HERA study (Piccart-Gebhart 2005; [2.5]), which was a more permissive
study in that it allowed a range of different chemotherapy regimens, and
women were simply randomized to trastuzumab or not at the completion of
their chemotherapy.
 DR LOVE: What did the data show? DR LOVE: What did the data show?
 DR WINER: The combined analysis showed a very impressive, highly statistically
significant improvement in disease-free survival for women who received
AC/paclitaxel plus trastuzumab, followed by trastuzumab, compared to those
women who received no trastuzumab whatsoever. The reduction in the risk of
recurrence was significant — in terms of the hazard ratio, there was a 40 to 50
percent reduction in the risk of disease recurrence. I’m purposely being a little
vague about the actual number, because it could change a little over time. I
don’t think we should get hung up as to whether it’s a 42 or 46 or 48 percent
reduction — the reduction was significant. DR WINER: The combined analysis showed a very impressive, highly statistically
significant improvement in disease-free survival for women who received
AC/paclitaxel plus trastuzumab, followed by trastuzumab, compared to those
women who received no trastuzumab whatsoever. The reduction in the risk of
recurrence was significant — in terms of the hazard ratio, there was a 40 to 50
percent reduction in the risk of disease recurrence. I’m purposely being a little
vague about the actual number, because it could change a little over time. I
don’t think we should get hung up as to whether it’s a 42 or 46 or 48 percent
reduction — the reduction was significant.
Moreover, there was also the early suggestion of a survival benefit, and what’s
particularly noteworthy is that this wasn’t a trial of trastuzumab early versus
no trastuzumab. It was a trial of trastuzumab early versus almost certainly
trastuzumab at the time of recurrence. I believe we have to presume that,
if not all, almost all of the women who developed metastatic breast cancer
received trastuzumab at that time.
The follow-up is very short from these trials — a couple of years or less — and
clearly, we need more follow-up. Whether this difference is maintained and
to what extent this leads to a long-term survival benefit remains to be seen,
although I think most people assume that there will be a significant survival
benefit as we move forward.
 DR LOVE: What about cardiac toxicity? DR LOVE: What about cardiac toxicity?
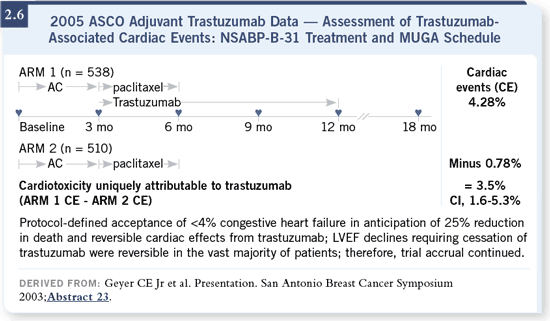
 DR WINER: The downside with receiving trastuzumab, apart from the fact
that it requires a year’s worth of therapy, is the cardiac toxicity, which was
defined as symptomatic congestive heart failure, so we’re not talking about
asymptomatic drops in ejection fractions. We’re talking about real problems
that we hope can improve over time, but about which we have relatively
limited, if any, information about the long-term consequences. I generally tell
patients that the risk of congestive heart failure is probably in the range of two
to four percent, based on what we know so far, specifically in women who
receive AC followed by paclitaxel with trastuzumab (2.6). DR WINER: The downside with receiving trastuzumab, apart from the fact
that it requires a year’s worth of therapy, is the cardiac toxicity, which was
defined as symptomatic congestive heart failure, so we’re not talking about
asymptomatic drops in ejection fractions. We’re talking about real problems
that we hope can improve over time, but about which we have relatively
limited, if any, information about the long-term consequences. I generally tell
patients that the risk of congestive heart failure is probably in the range of two
to four percent, based on what we know so far, specifically in women who
receive AC followed by paclitaxel with trastuzumab (2.6).
There was some suggestion that the cardiac toxicity may be less when trastuzumab
is administered sequentially, as in the NCCTG trial where paclitaxel
was given and then trastuzumab followed (Perez 2005b; [2.7]). Maybe that relates to a longer period of time from when the anthracycline is given to
the beginning of trastuzumab. There was also the suggestion of less cardiac
toxicity in the HERA trial, where chemotherapy and trastuzumab were not
concurrent (Piccart-Gebhart 2005).
In the NSABP analysis, there was the suggestion that cardiac toxicity was
more of a problem in older women, specifically in women who had borderline
ejection fractions at baseline versus those who had better, stronger, higher
ejection fractions. All of this needs to be sorted out.
 DR LOVE: There were some cardiac safety data from the BCIRG trial
presented by Dennis Slamon at ASCO. Can you talk about that? DR LOVE: There were some cardiac safety data from the BCIRG trial
presented by Dennis Slamon at ASCO. Can you talk about that?
 DR WINER: In the BCIRG trial, in the group of women who received
docetaxel, carboplatin and trastuzumab, the cardiac toxicity was substantially
less than in women who received the anthracycline followed by docetaxel
and trastuzumab. I think all of us are very hopeful that nonanthracyclinecontaining
regimens will be the wave of the future, but we will just have to
wait for the efficacy data. We need those data from the BCIRG trial, and
I’m told that we will have those some time over the next several months
— certainly at San Antonio, if not before. DR WINER: In the BCIRG trial, in the group of women who received
docetaxel, carboplatin and trastuzumab, the cardiac toxicity was substantially
less than in women who received the anthracycline followed by docetaxel
and trastuzumab. I think all of us are very hopeful that nonanthracyclinecontaining
regimens will be the wave of the future, but we will just have to
wait for the efficacy data. We need those data from the BCIRG trial, and
I’m told that we will have those some time over the next several months
— certainly at San Antonio, if not before.

 Track 11 Track 11
 DR LOVE: What can we say about the effects of adjuvant trastuzumab in
patients with node-negative tumors in terms of clinical practice at this
point? How are you going to approach patients in your practice with
HER2-positive, node-negative disease? DR LOVE: What can we say about the effects of adjuvant trastuzumab in
patients with node-negative tumors in terms of clinical practice at this
point? How are you going to approach patients in your practice with
HER2-positive, node-negative disease? |
 DR WINER: In the HERA study (Piccart-Gebhart 2005), they included
patients with node-negative disease as long as their tumors were greater than
a centimeter. A third of the patients participating were node-negative, but we
don’t know how many of the events occurred in those patients. DR WINER: In the HERA study (Piccart-Gebhart 2005), they included
patients with node-negative disease as long as their tumors were greater than
a centimeter. A third of the patients participating were node-negative, but we
don’t know how many of the events occurred in those patients.
The NSABP trial had no patients with node-negative disease, and in the
NCCTG study, patients with node-negative disease accounted for 14 percent
of the total population but only six percent of the events (Romond 2005).
I think it’s unlikely that the relative benefits of trastuzumab will be different
in patients with node-negative versus node-positive disease. On the other
hand, the absolute benefit will differ, because patients with node-negative
disease, particularly with small tumors, have a lower risk of recurrence.
In my mind, it’s reasonable to consider trastuzumab for patients who were
eligible for the studies. The group of women that I’m a little more cautious
about are those with relatively small, ER-positive, node-negative breast cancer.
I realize that in the HERA study, a woman with a one- to two-centimeter,
ER-positive, node-negative cancer could have been included, but I don’t think
we have a sense as to the benefit of trastuzumab in that patient.
I’m not entirely clear what that woman’s risk of disease recurrence would
be, particularly with modern hormonal therapy. I’m not saying that woman
shouldn’t receive trastuzumab, but I would say that we have to be a little more
cautious when we’re talking about the lower-risk patients, since they are at
equal risk as the higher-risk patients to experience toxicities.
SELECT PUBLICATIONS
|

