 |
| I N T E R V I E W |
 |
|
| Kathy D Miller, MD |
Dr Miller is the Sheila D Ward Scholar of Medicine and
Associate Professor of Medicine in the Department of
Hematology/Oncology at Indiana University School of
Medicine in Indianapolis, Indiana. |
 |
|
|
- For most patients with metastatic disease, is sequential
single-agent chemotherapy more appropriate than
combination chemotherapy?
- Is bevacizumab combined with paclitaxel or capecitabine
generally the preferred first-line chemotherapy for patients with
metastatic disease?*
|
 On the question of the use of a sequential
single agent over combination chemotherapy
for most patients with metastatic disease,
it will be no surprise to most of you that I
would strongly agree. On the question of the use of a sequential
single agent over combination chemotherapy
for most patients with metastatic disease,
it will be no surprise to most of you that I
would strongly agree.
These are the reasons why I would agree that
single-agent chemotherapy in
a sequential fashion should be
the preferred choice for the
vast majority of our patients.

Slide 1
This question has been
tackled in different ways.
These three separate trial
designs really don’t ask the
same question — they ask
two different questions. These
first two trial designs ask, Is a
particular drug beneficial in
breast cancer?
The third design, which
includes a sequential strategy versus a combination strategy, asks how to best use drugs that we already
know or assume are active.
Slide 2
This is a trial that is familiar to you — the docetaxel/capecitabine trial with
the doses and schedules that you see here. Only about 20 percent of patients
crossed over, so this is not a trial that asked, How do we best use capecitabine
in our patients? It’s a trial that asked, Is capecitabine beneficial?
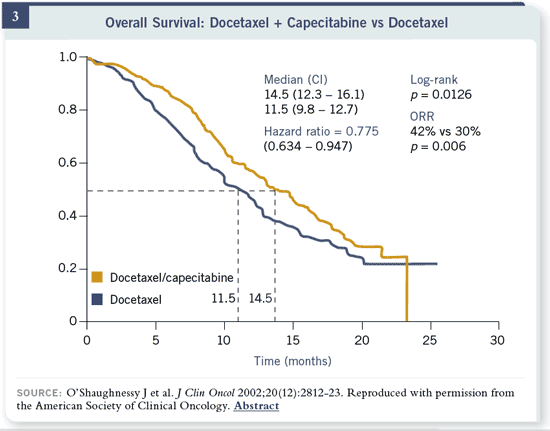
Slide 3
It clearly is. Those patients who received capecitabine had a much higher
response rate, better time to progression and improvement in overall survival.
Slide 4
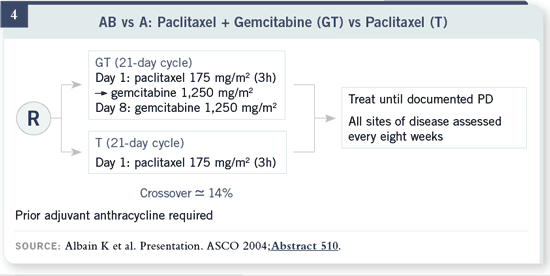
There’s a similar trial design with gemcitabine with about 15 percent crossover.
The question is the same: Is gemcitabine beneficial in this disease? The
question is not, How do we best use gemcitabine in our patients?
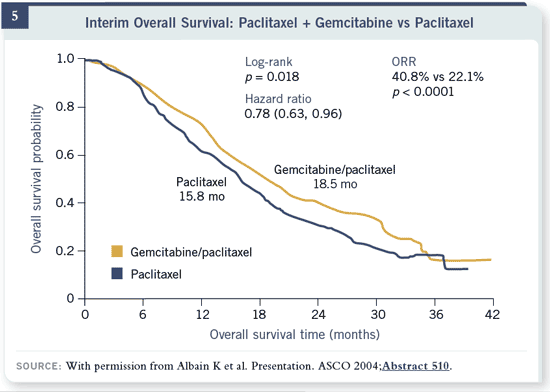
Slide 5
The results were very similar with improvements in overall survival and
response rates. In both of these trials, there was increased toxicity in the
combination arm and no difference in overall survival. You could argue that
the slight increase in response rates is discounted, in a quality-of-life sense, by
the increase in toxicities.
Slide 6
There have been many other trials that have had similar designs. These trials
go back to the 1970s, and there were only three that found improvement in
overall survival. What is striking about all three of them is that they coincide
with the introduction of a new therapy that we have all come to accept as one
that is highly active and beneficial for at least some of our patients.
There are several trials that have looked at the strategy question rather than
the drug question.
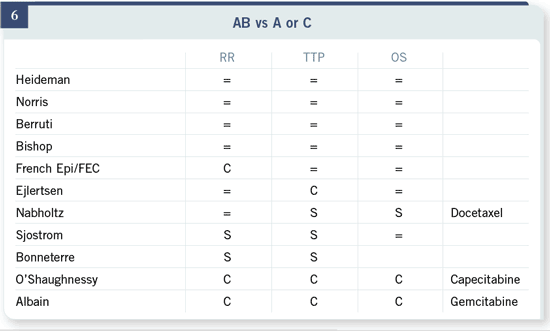
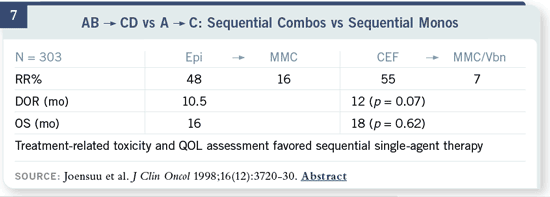
Slide 7
This is a trial from our European colleagues that looked not only at single
drugs but also whether sequential single agents versus sequential combinations
— keeping the single agents in those combinations — are beneficial. There
were no improvements in any of the endpoints with the combination therapy
regimen, and treatment-related toxicity and quality of life certainly favored
the single agents.
Slide 8
The largest trial and perhaps the one that has been the hallmark for this
particular question is George Sledge’s E1193 trial using doxorubicin and paclitaxel
as either single agents or in combination, with a planned crossover to the
other agent in patients randomized to the single-agent arms.
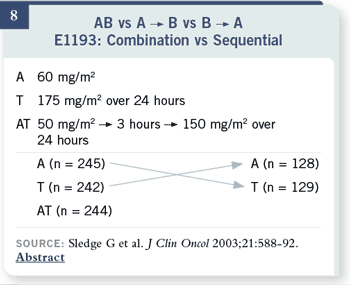
Slide 9
On this slide, I have tried to
summarize all of the results
that I think are important
for this trial. As initial
therapy, there were slightly
greater response rates
and about a two-month
improvement in time to
progression for the combination,
but there was no
difference in overall survival
or quality of life. In the
crossover results, response
rates, time to progression
and overall survival at the
time of second-line therapy
were also essentially equivalent.
With these particular
agents, we couldn’t define a
superior
sequence.
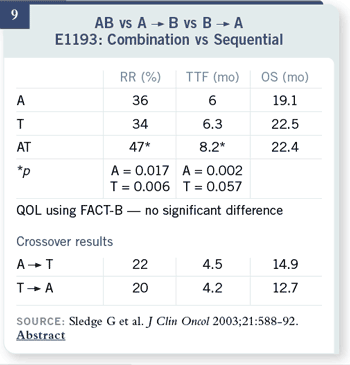
Slide 10
There are several other
trials, going back to the
1970s, which have used this
particular trial design. The
Baker trial actually included
five chemotherapy agents,
either given all together or
in sequence. The results are
markedly consistent. There
was no difference in overall
survival in any of
these efforts.
Slide 11
We have to go back to
what our therapy goals for
metastatic disease are. This
is what my patients tell me.
This is not really what I say.
My patients tell me they
wish to live longer and better during that time, however long or short it
might be.
Our clinical trials always
tell us about response rates.
They may tell us about
duration of response or time
to progression. Occasionally,
they tell us about overall
survival, though these timeto-
event variables in uncontrolled
trials are very hard
to dissect and interpret.
And they often either don’t
assess quality of life, or our
quality-of-life tools are fairly
crude and only tell us about
large differences.
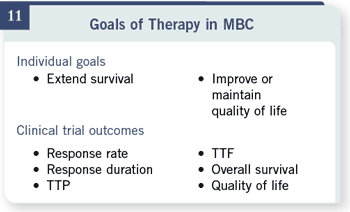 We have fairly good data that
tell us that those clinical trial
results, other than overall
survival, really don’t translate
very well into extending
survival and improving or
maintaining quality of life. We have fairly good data that
tell us that those clinical trial
results, other than overall
survival, really don’t translate
very well into extending
survival and improving or
maintaining quality of life.
Slide 12
That is why, in my opinion, for most patients, the sequential single-agent
approach is preferred — it gives us a variety of options.
 From this year’s ASCO, several other trials are looking at whether the order of
those agents makes a difference. We have yet to identify any particular order
that, overall, gives patients greater benefit than a different order. So there are
many options that allow us to individualize both treatment options and the
order of treatment for our patients, based on what our patients bring to us. From this year’s ASCO, several other trials are looking at whether the order of
those agents makes a difference. We have yet to identify any particular order
that, overall, gives patients greater benefit than a different order. So there are
many options that allow us to individualize both treatment options and the
order of treatment for our patients, based on what our patients bring to us.
For those patients we all talk about but rarely see who have such symptomatic,
rapidly progressive disease that you think — if they don’t respond to the
first treatment option, you’re not going to get another chance in four, six or
eight weeks — a combination is certainly appropriate. I just don’t think that
happens very often, certainly not in the first-line setting. Usually, when I see
patients in that situation, they’ve already had much previous chemotherapy, so
my options are already limited.
- Is bevacizumab combined with paclitaxel or capecitabine generally
the preferred first-line chemotherapy for patients with metastatic
disease?
|
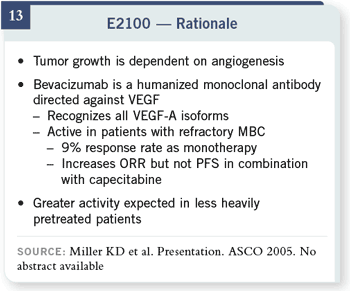
Slide 13
E2100 is based on a different
hypothesis. It really is not
asking a chemotherapy
question. It’s based on our
understanding that angiogenesis
is important in breast
cancer. There are many proangiogenic
factors that are
produced by breast cancer,
but the vascular endothelial
growth factor (VEGF)
is one of the most common
and potent ones.
We do know that bevacizumab
is an agent that
has some activity in breast
cancer but is not particularly
great in refractory
patients, with only about a
nine percent response rate
as monotherapy. This was a
slight improvement in the
response rate, but progression-
free or overall survival
did not improve in those
patients.

The hypothesis that E2100
was designed to test was
based on the biology that
using this sort of agent
earlier in the course of the
disease would give greater activity.
Slide 14
This trial randomized patients to paclitaxel with or without bevacizumab. A
fairly common four-week treatment cycle was used, with a schedule of weekly
paclitaxel for three weeks and then one week off. Patients were eligible if they
had metastatic or locally recurrent disease that was not curable with any local
therapy approach.
Slide 15
 Essentially, this is a trial
for patients with HER2-
negative disease, because
the trial schema does
not include trastuzumab.
Patients with HER2-
positive disease were only
allowed into this trial if
they had received previous
trastuzumab therapy. Note
that patients couldn’t have
received previous chemotherapy
for metastatic
disease. We thought this
could apply to someone who
was in one of the adjuvant
trastuzumab studies and
progressed fairly soon after
her trastuzumab therapy
or someone who received
trastuzumab monotherapy
for metastatic disease and
then wanted to enter this
trial. In reality, there were
only a handful of patients
with HER2-positive
disease, and they all came
from South Africa, where
trastuzumab is not available.
Otherwise, patients had
to be healthy and, Essentially, this is a trial
for patients with HER2-
negative disease, because
the trial schema does
not include trastuzumab.
Patients with HER2-
positive disease were only
allowed into this trial if
they had received previous
trastuzumab therapy. Note
that patients couldn’t have
received previous chemotherapy
for metastatic
disease. We thought this
could apply to someone who
was in one of the adjuvant
trastuzumab studies and
progressed fairly soon after
her trastuzumab therapy
or someone who received
trastuzumab monotherapy
for metastatic disease and
then wanted to enter this
trial. In reality, there were
only a handful of patients
with HER2-positive
disease, and they all came
from South Africa, where
trastuzumab is not available.
Otherwise, patients had
to be healthy and,
significantly,
patients with CNS
metastases
were excluded.
We did require screening
before entry.
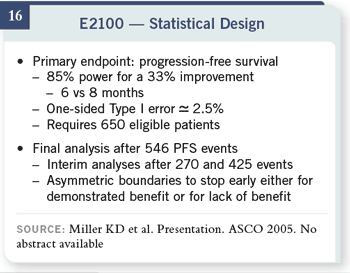
Slide 16
We had fairly modest goals
for this trial. We were
hoping to find an improvement
in progression-free
survival from six to eight
months in the paclitaxel-alone arm. That would have required 650 patients.
There were planned interim analyses that used very stringent O’Brien-
Fleming boundaries to release the results early, either because there was a clear benefit or a definite lack
of benefit.
Slide 17
 The results that we have
now are from the first
planned interim analysis.
The trial accrued 715
eligible patients over about
two and a half years. The
data is now current through
February 9th of this year and
includes 355 events, most
of which are progression.
The 64 patients who were
censored at death without
progression had progressive
disease in most of the
situations — but there was
not adequate imaging or
documentation to meet
RECIST criteria for
disease progression. The results that we have
now are from the first
planned interim analysis.
The trial accrued 715
eligible patients over about
two and a half years. The
data is now current through
February 9th of this year and
includes 355 events, most
of which are progression.
The 64 patients who were
censored at death without
progression had progressive
disease in most of the
situations — but there was
not adequate imaging or
documentation to meet
RECIST criteria for
disease progression.
Slide 18
Patients were well matched.
They were the usual age for
a breast cancer trial — in
their mid-fifties, though
there were patients enrolled
who were in their mid-eighties.
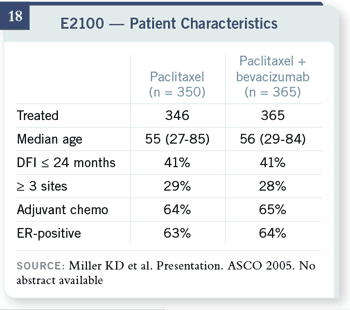 Two thirds of the patients
in this trial had received
adjuvant chemotherapy,
which is much higher than
previous metastatic trials.
That tells us that how we
treat these patients has
shifted, so you can’t easily
compare results of first-line
trials done today to results
of first-line trials done 10
years ago. Two thirds of the patients
in this trial had received
adjuvant chemotherapy,
which is much higher than
previous metastatic trials.
That tells us that how we
treat these patients has
shifted, so you can’t easily
compare results of first-line
trials done today to results
of first-line trials done 10
years ago.
The response data are
the first encouraging bit
of news, and this is my
evidence that we can
evaluate response in patients
without measurable disease
quite well.
Slide 19
E2100 did not require
measurable disease because
progression-free survival
was the primary endpoint.
As the PI, I didn’t care if
you could tell me if the
patient was responding,
but I was fairly certain that
good clinicians would know
when their patients were
progressing, and that was the endpoint we were interested in.
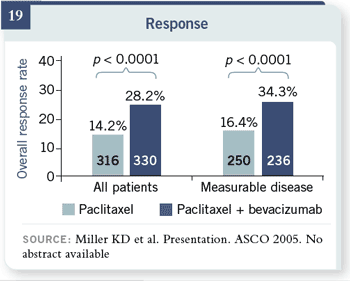 If you look at the subset of about three quarters of patients who had measurable
disease, the estimates of response were very similar. If you look at the subset of about three quarters of patients who had measurable
disease, the estimates of response were very similar.
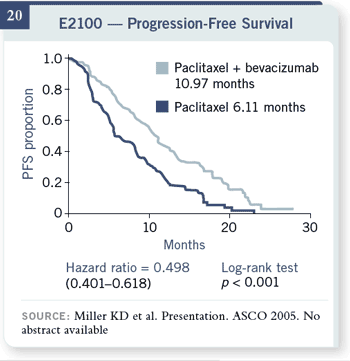
Slide 20
Progression-free survival was clearly improved by much greater than we
had expected. Our estimates of a six-month progression-free survival for
patients receiving paclitaxel alone was 6.1 months, with an improvement to
10.97 months or a 51 percent decrease in the risk of progression for patients
receiving the combination.
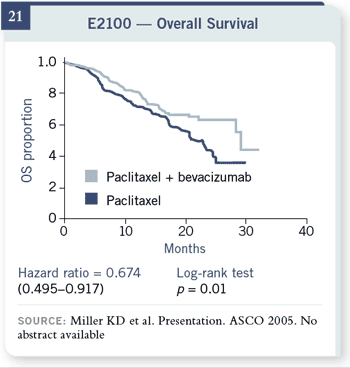
Slide 21
 The overall survival data,
I must caution you, are
early, so I cannot tell you
what the median survivals
are. They’re just now being
reached, and that part of
the curve is very unstable.
What is not likely to
change, however, is that
there is a survival advantage
in this trial, with about a
33 percent decrease in the
risk of death in patients
receiving the combination
therapy. The overall survival data,
I must caution you, are
early, so I cannot tell you
what the median survivals
are. They’re just now being
reached, and that part of
the curve is very unstable.
What is not likely to
change, however, is that
there is a survival advantage
in this trial, with about a
33 percent decrease in the
risk of death in patients
receiving the combination
therapy.
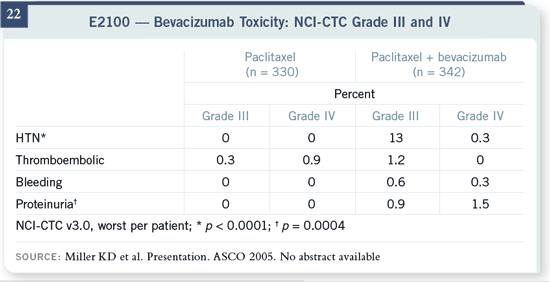
Slide 22
Increase in toxicity was minimal. We’ve known about the bevacizumabassociated
toxicities, and this is about what we expected. About 15 percent of
patients developed hypertension and needed treatment, and there was a small
proportion of patients with significant proteinuria without any other signs of
renal dysfunction. In this relatively healthy breast cancer patient population,
thromboembolic events and bleeding were not a problem, and they were not
different in the two treatment groups.
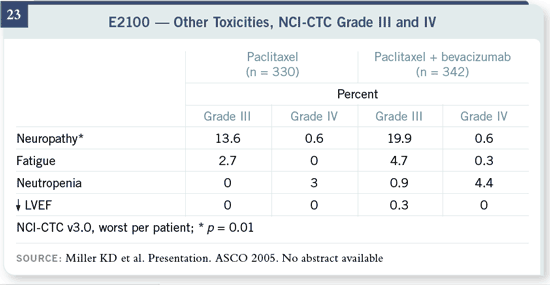
Slide 23
There’s only one important difference in the chemotherapy-associated toxicity,
which is a slight increase in the risk of Grade III neuropathy. It could be that
there’s an interaction between the drugs that increases neuropathy. What’s more likely is, since paclitaxel-related neuropathy is both duration of treatment
and dose related, patients were responding to therapy for a much longer time
and were exposed to a greater duration of taxane therapy.
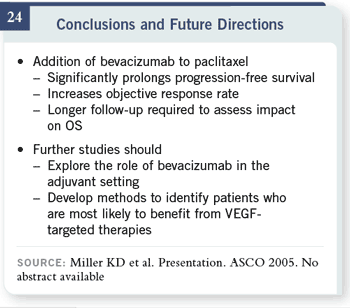
Slide 24
Overall, we were delighted to be able to finally say that the addition of
bevacizumab in patients with newly diagnosed metastatic breast cancer — or
at least newly requiring chemotherapy for their metastatic breast cancer
— improves the progression-free survival and the objective response rate. It
also appears to improve overall survival, though we’ll need further follow-up
to know the magnitude of improvement in overall survival.
We are continuing to look for ways to identify those patients who are most
likely to benefit from this approach. These patients were not positively selected
for any molecular feature because, at this point, we don’t know how to
select them.
Slide 25
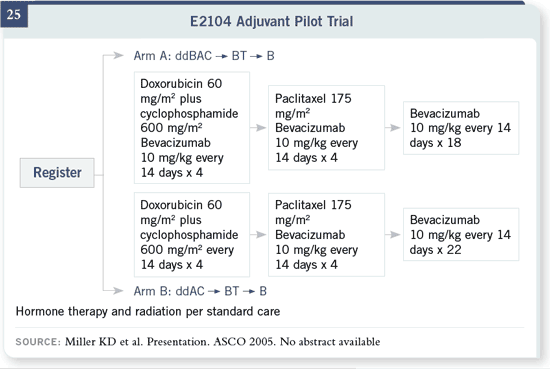
We’re also looking forward to exploring bevacizumab in the adjuvant setting
and will very soon be activating an adjuvant pilot trial in the Eastern Cooperative
Oncology Group. This trial will hopefully then be followed, in very
quick succession, with a full adjuvant trial to try and look for improvements in
disease-free and overall survival.
- Is bevacizumab combined with paclitaxel or capecitabine
generally the preferred first-line chemotherapy for patients with
metastatic disease?
|
I would agree with this second question as well. For most patients, weekly
paclitaxel or capecitabine in combination with bevacizumab provides the most
effective first-line chemotherapy. The trial I’ve just shown you, E2100, clearly
finds significant improvements in all of those response parameters for incorporating
bevacizumab with weekly paclitaxel.
 It’s important to think about the eligibility criteria and which of our patients
with metastatic disease would not have been eligible for this trial. We don’t
yet know about the role of bevacizumab or the safety of bevacizumab in
combination with trastuzumab regimens in patients who are HER2-positive.
At this point, I would not add bevacizumab to those patients’ treatment. We
also excluded those patients who had received adjuvant taxane therapy and
progressed within 12 months. I don’t think it would be reasonable to re-treat
those patients with paclitaxel, even in combination with bevacizumab. So for
those patients who’ve recently had an adjuvant taxane and are progressing, we
really don’t have good data.
It’s important to think about the eligibility criteria and which of our patients
with metastatic disease would not have been eligible for this trial. We don’t
yet know about the role of bevacizumab or the safety of bevacizumab in
combination with trastuzumab regimens in patients who are HER2-positive.
At this point, I would not add bevacizumab to those patients’ treatment. We
also excluded those patients who had received adjuvant taxane therapy and
progressed within 12 months. I don’t think it would be reasonable to re-treat
those patients with paclitaxel, even in combination with bevacizumab. So for
those patients who’ve recently had an adjuvant taxane and are progressing, we
really don’t have good data.
 From our previous
capecitabine trials, I can tell
you that those patients who
receive an anthracycline and
taxane and progress within
12 months do miserably,
and I suspect they would do
miserably no matter what
we did for them. If you are
interested, it’s reasonable to
use bevacizumab in those
patients, but we don’t have
randomized trial data to
support that at this point.
From our previous
capecitabine trials, I can tell
you that those patients who
receive an anthracycline and
taxane and progress within
12 months do miserably,
and I suspect they would do
miserably no matter what
we did for them. If you are
interested, it’s reasonable to
use bevacizumab in those
patients, but we don’t have
randomized trial data to
support that at this point.
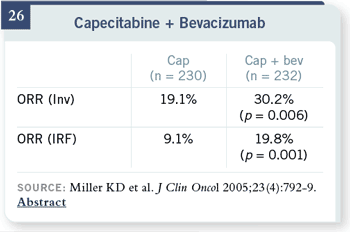
Slide 26
We have safety and response
data with bevacizumab
in combination with
capecitabine in exactly
that patient population.
Response rates increased
slightly, but most of those
extra responses were fairly
short lived.
Slide 27
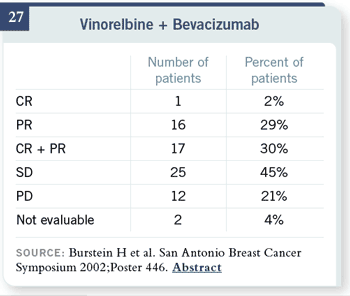
 There are also safety and
response data in patients
who were pretreated with
vinorelbine. Either one of
those would be regimens for
which at least there is some
evidence, although more on
safety than on efficacy. There are also safety and
response data in patients
who were pretreated with
vinorelbine. Either one of
those would be regimens for
which at least there is some
evidence, although more on
safety than on efficacy.
Slide 28
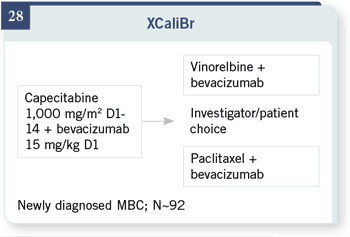
Finally, there is another trial
that will be starting very soon called XCaliBr. This trial will look at newly
diagnosed patients, essentially the same group as in the E2100 trial — needing
chemotherapy, but using capecitabine in combination with bevacizumab. This
trial allows but does not require patients to continue bevacizumab after initial
progression, either with vinorelbine or paclitaxel, at the patients’ and investigators’
choice.
This is a fairly small Phase II trial with only 92 patients, so it certainly will
not be definitive. Randomization to continuing bevacizumab or not is not
included. That is an open question we need to address quickly. But the trial
will, at least, give us some comparative data on response rates and time-toevent
variables, with a different chemotherapy regimen. That will be particularly
helpful as the taxanes are used more and more in the adjuvant setting and
as we hope to move to more oral therapies and therapies that don’t include
alopecia and some of those toxicities for our patients with metastatic disease.
SELECT PUBLICATIONS
* Presented at Research To Practice Breast Cancer Update CME Forum, Los Angeles, California,
May 21, 2005. The enclosed graphics from the presentation are included in the PowerPoint files on CD 3.
Please see page 1 for additional instructions.
|

