 |
| PRESENTATION |
 |
|
| Rowan T Chlebowski, MD, PhD |
Dr Chlebowski is a Professor of Medicine at UCLA
School of Medicine and Chief of Medical Oncology at
Harbor-UCLA Medical Center in Torrance, California. |
 |
|
|
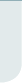
- Update of the Women's Intervention Nutrition Study (WINS) trial
- Overview of Adjuvant Hormonal Therapy Trials: Comparing and
Sequencing Tamoxifen with Aromatase Inhibitors*
|
Slide 1
 I am going to talk a little
about the WINS trial that
was reported at ASCO in a
plenary session. Women in
this study were 48 to 79 years
of age, mostly postmenopausal
with early-stage breast
cancer, Stage I to IIIA, and
had received primary surgery,
radiation therapy and conventional
systemic therapy.
Patients with receptorpositive
tumors received
tamoxifen, while those with
receptor-negative tumors
received chemotherapy —
half anthracycline based and
half nonanthracycline based.
The women on tamoxifen
could have elected to receive
chemotherapy as well. The
patients were randomized
60:40, with fewer to the
dietary intervention group, so we would have more resources to allocate to the
dietary intervention group or control.
I am going to talk a little
about the WINS trial that
was reported at ASCO in a
plenary session. Women in
this study were 48 to 79 years
of age, mostly postmenopausal
with early-stage breast
cancer, Stage I to IIIA, and
had received primary surgery,
radiation therapy and conventional
systemic therapy.
Patients with receptorpositive
tumors received
tamoxifen, while those with
receptor-negative tumors
received chemotherapy —
half anthracycline based and
half nonanthracycline based.
The women on tamoxifen
could have elected to receive
chemotherapy as well. The
patients were randomized
60:40, with fewer to the
dietary intervention group, so we would have more resources to allocate to the
dietary intervention group or control.
Slide 2
Randomization took place about 220 days after initial surgery, so patients
were entered after they completed their primary therapy, while they were
receiving hormonal therapy. The dietary intervention was done at 37 centers
around the United States.
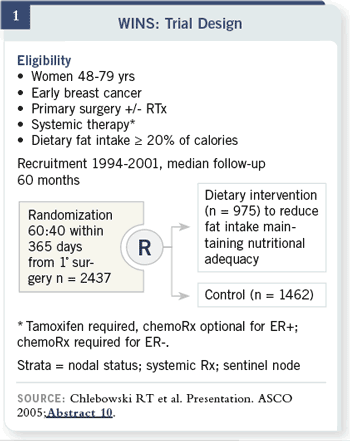
The diet group was given a dietary fat gram goal by centrally trained, registered
dieticians, implementing a predefined low-fat eating plan. The dieticians were trained in behavioral intervention techniques. Patients received eight
biweekly individual counseling sessions then one session every three months.
There was no counseling towards weight reduction.
This was more of a switching trial than a weight-reduction trial. There
were monthly group sessions, and patients self-monitored their fat intake.
We captured information about their diet with telephone re-calls. The
control group saw the dieticians every three months and talked about
nutritional adequacy.
Fat gram intake for this group went from about 56 to 33 fat grams per day —
about a 40 percent reduction in daily fat gram intake — which was sustained
by most of the individuals.
There was a four-pound weight loss, which wasn’t much but still was three
standard deviations different. The weight loss at least signals that some dietary
change had occurred.
Slide 3
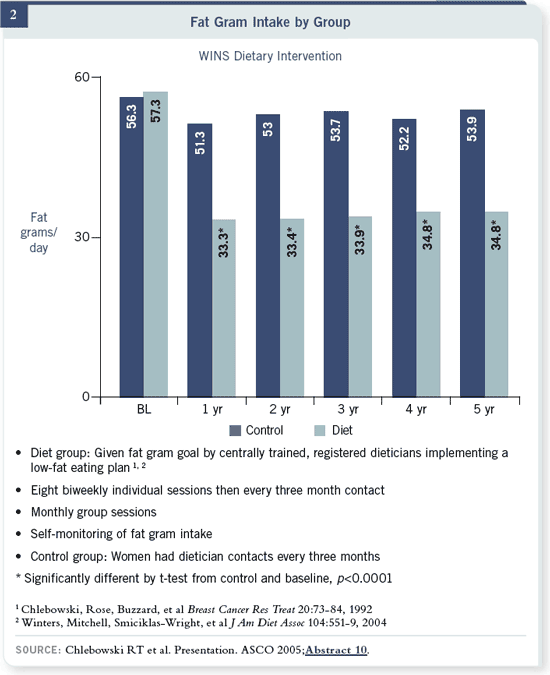
Here is our primary study endpoint of WINS relapse-free survival. This is an
interim result, as the follow-up is continuing. As you can see, the hazard ratio
was 0.76 with a p-value of 0.034 for Cox proportional hazard, and there was a
three percent difference at five years.
We did a subgroup analysis by receptor status. The hazard ratio for relapse-free
survival for patients with estrogen receptor-positive tumors was 0.85 and not
significant. In the 478 patients with ER-negative disease, there was a hazard
ratio of 0.58, with a 42 percent reduction in risk and eight percent absolute
difference at five years. This is hypothesis generating but very intriguing to us,
as the curves just break apart initially.
- Overview of Adjuvant Hormonal Therapy Trials: Comparing and
Sequencing Tamoxifen with Aromatase Inhibitors
|
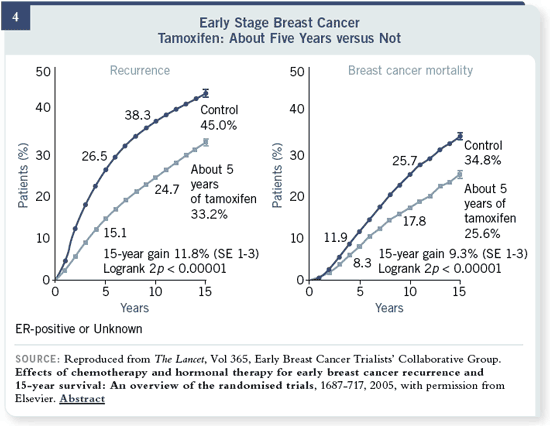
Slide 4
 These are data from the
Early Breast Cancer Trialists’
Collaborative Group, which
is now published and available
online. You can see that
there was a 14 or 15 percent
reduction in recurrence risk
with tamoxifen. To the right,
this is mortality risk going
forward. That is why tamoxifen
has been the standard
therapy for all these years. These are data from the
Early Breast Cancer Trialists’
Collaborative Group, which
is now published and available
online. You can see that
there was a 14 or 15 percent
reduction in recurrence risk
with tamoxifen. To the right,
this is mortality risk going
forward. That is why tamoxifen
has been the standard
therapy for all these years.
Slide 5
We also know that there are life-threatening toxicities for tamoxifen —
endometrial cancer, pulmonary embolus, stroke. It took us 20 years to figure
out that tamoxifen had an endometrial cancer risk. But we also should say
that if we did 32 trials of tamoxifen versus nothing, it still took us 15 years to figure out that tamoxifen worked. So I think that if a trial has 3,000 or 4,000
patients in each arm, we’ll get to a toxicity issue sooner.
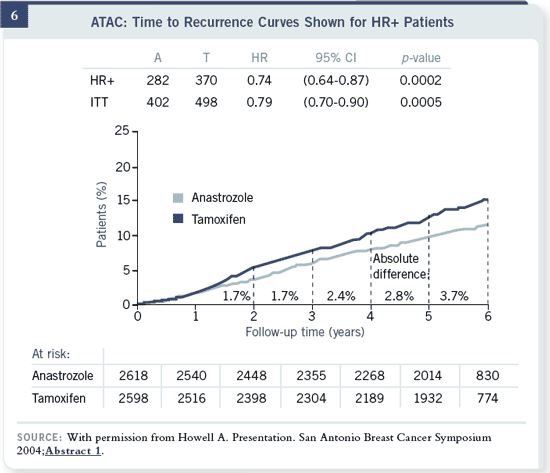
Slide 6
You’ve seen this before; the only point I want to make about the ATAC data
is that they’re at 68 months. That means that almost a year of follow-up has
occurred for people who have stopped their aromatase inhibitors.
So when we look at the toxicity of the ATAC trial, this is what it will be.
There aren’t any studies for more than five years of aromatase inhibitors.
People say, “I’m worried about long-term use of aromatase inhibitors.” Well,
this should be the toxicity that you’re going to see, because it’s five years
of toxicity.
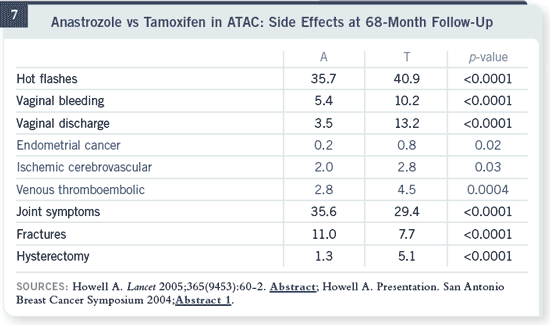
Slide 7
 These are the updated data on side effects. At the 68-month follow-up, half
the patients have been off therapy for nearly a year, and we saw the expected
reduction in all three life-threatening toxicities of endometrial cancer, stroke
and venous thromboembolic disease. You can see all of them were significant. These are the updated data on side effects. At the 68-month follow-up, half
the patients have been off therapy for nearly a year, and we saw the expected
reduction in all three life-threatening toxicities of endometrial cancer, stroke
and venous thromboembolic disease. You can see all of them were significant.
Slide 8
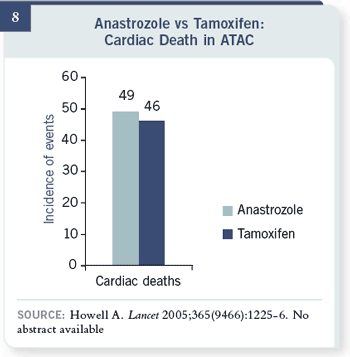
There are 3,000 patients in
each arm. Cardiac deaths
were 49 versus 46 — so there
wasn’t much of a signal for
myocardial infarction issues
with anastrozole — at least
with the data from the 68-
month follow-up from the
ATAC trial.
Slide 9
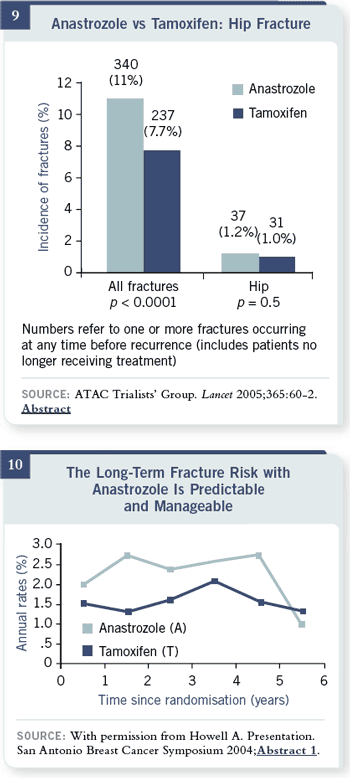 The other point I want to
make is that many oncologists
have a lot of concern
regarding bones. I think
it’s going to be not only a
preventable, treatable situation
but also something that
is likely to go away completely in the near future. There is no difference in
hip fractures after 68 months with anastrozole and tamoxifen. You can see
that hip fracture is one percent — a tenth of a percent per year. This is for a
group of patients who had no prescreening when they entered the study and
no ongoing protocol-defined follow-up for bone. These are high numbers. If
you’re going to actually do any screening, any treating, you’re going to have
lower numbers than that. The hip fractures are the ones that are associated
with a survival detriment.
The other point I want to
make is that many oncologists
have a lot of concern
regarding bones. I think
it’s going to be not only a
preventable, treatable situation
but also something that
is likely to go away completely in the near future. There is no difference in
hip fractures after 68 months with anastrozole and tamoxifen. You can see
that hip fracture is one percent — a tenth of a percent per year. This is for a
group of patients who had no prescreening when they entered the study and
no ongoing protocol-defined follow-up for bone. These are high numbers. If
you’re going to actually do any screening, any treating, you’re going to have
lower numbers than that. The hip fractures are the ones that are associated
with a survival detriment.
Slide 10
Here are the fracture rates
from the update of the
ATAC data presented by
Tony Howell. You can see
that at the five-and-a-halfyear
point, the fracture rates
look like they’re converging.
For hormonal therapy,
we know that when you
stop estrogen/progestin or
estrogen alone, bone loss
is accelerated. It’s two to
three times the rate of loss in
normal menopause. Will we
get accelerated bone recovery
when we add back the
hormones? We’ll know that
soon from the bone density
studies that are ongoing.
There may not be long-term
consequences of five years of
aromatase inhibition.
Slide 11
The other issue is cognition.
A few years ago, we thought
that preclinical observational
studies suggested that
exogenous estrogens and
lowering of progestins would
be important in maintaining
cognition. There were trials
started in patients with
dementia. The Women’s
Health Initiative studies that
I’ve been involved with have
totally changed that concept.
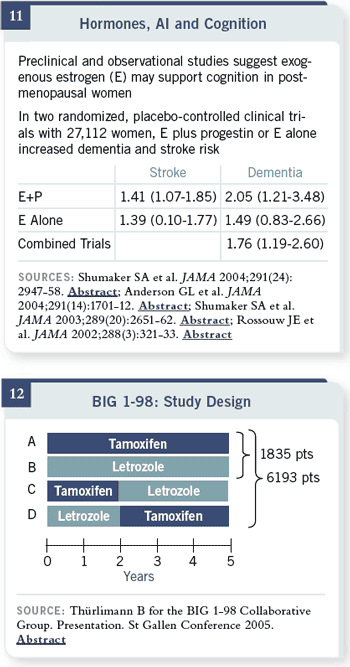 In two randomized, placebo-controlled clinical trials, 16,000 otherwise
healthy women received estrogen plus progestin therapy for five years, and the
dementia was only two and a half years, but there was a 40 percent increase in
stroke and doubling of dementia risk in a subset of patients 65 years of age or
older who received estrogen/ progestin versus placebo for just two and a half
years. In the estrogen-alone trial, there was a 40 percent increase in stroke
and a 50 percent increase in dementia. A preplanned combined analysis had a statistically significant 76
percent increase in dementia.
So the old idea is that exogenous
estrogens maintain
cognition. The new concept
is that anything that causes
arterial vascular events —
like estrogen, estrogen plus
progestin, maybe tamoxifen
— will be likely to increase
not only stroke but also
decrease brain function and
increase the risk of dementia.
We don’t know what aromatase
inhibitors will do to
cognition, but the testing
has to be two tailed, because
one could equally say that it
might have favorable impacts
on cognition.
In two randomized, placebo-controlled clinical trials, 16,000 otherwise
healthy women received estrogen plus progestin therapy for five years, and the
dementia was only two and a half years, but there was a 40 percent increase in
stroke and doubling of dementia risk in a subset of patients 65 years of age or
older who received estrogen/ progestin versus placebo for just two and a half
years. In the estrogen-alone trial, there was a 40 percent increase in stroke
and a 50 percent increase in dementia. A preplanned combined analysis had a statistically significant 76
percent increase in dementia.
So the old idea is that exogenous
estrogens maintain
cognition. The new concept
is that anything that causes
arterial vascular events —
like estrogen, estrogen plus
progestin, maybe tamoxifen
— will be likely to increase
not only stroke but also
decrease brain function and
increase the risk of dementia.
We don’t know what aromatase
inhibitors will do to
cognition, but the testing
has to be two tailed, because
one could equally say that it
might have favorable impacts
on cognition.
Slide 12
The BIG 1-98 study is our
only switching study. We
probably won’t get results
from it for several years,
and they may not be definitive.
These are big studies,
with 8,000 patients, but the
switching parts — tamoxifen/
letrozole, letrozole/
tamoxifen — are half that
size. There are 1,250 patients
in each of those arms,
numbers that would be small
for switching.
Slide 13
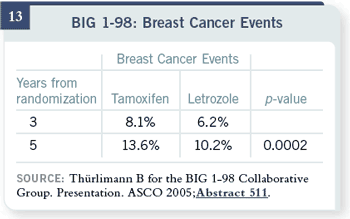 Here are data similar to the
ATAC data — 13.6 percent
rate of relapse with tamoxifen
versus 10.2 percent with
letrozole — about the same
difference after this relatively
short period of follow-up.
Here are data similar to the
ATAC data — 13.6 percent
rate of relapse with tamoxifen
versus 10.2 percent with
letrozole — about the same
difference after this relatively
short period of follow-up.
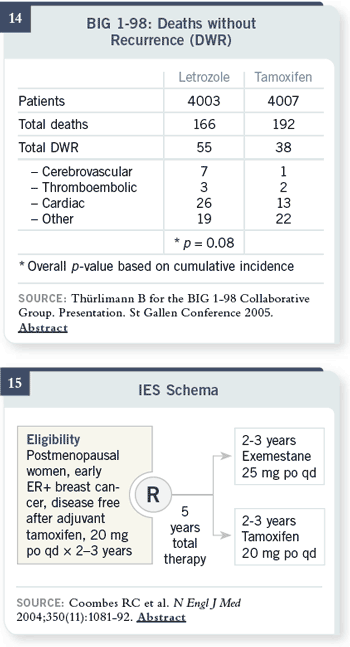
Slide 14
Here are the data for deaths,
cardiac — 26 with letrozole
versus 13 with tamoxifen.
You didn’t see anything at all
like this in the MA17 trial,
comparing letrozole versus
placebo after tamoxifen —
there was absolutely no signal
of cardiac issue there.
 It was alluded to that the
population is different, but it
actually isn’t that different.
It is 15 percent Eastern
European. Almost all the
rest, 85 percent, is Western
European, but there is no
US, and the UK is underrepresented
compared to the
other trials.
It was alluded to that the
population is different, but it
actually isn’t that different.
It is 15 percent Eastern
European. Almost all the
rest, 85 percent, is Western
European, but there is no
US, and the UK is underrepresented
compared to the
other trials.
The population is mostly
Western European, so there
was no great explanation for
that difference.
Slide 15
Now we’ll go to the
switching trials. This is
the IES trial, which is
exemestane versus tamoxifen
after two to three years
of tamoxifen.
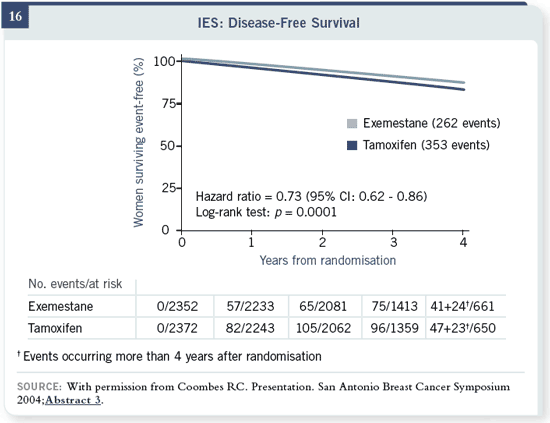
Slide 16
You can see with this recent update that there was a 27 percent reduction in
recurrence risk. And there was a trend towards a survival benefit, which we
didn’t see in the ATAC trial. We’ll get into that later in terms of what that
would mean when you’re switching in the middle and randomizing in the
middle versus randomizing at the start. So there was a 20 to 27 percent reduction
in risk of recurrence and a trend toward survival — but there were more
myocardial infarctions on exemestane.
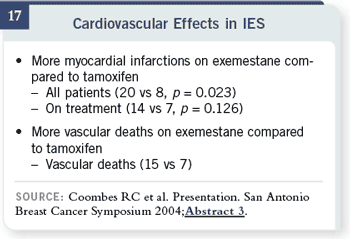
Slide 17
These were small numbers
— 20 with exemestane
versus eight with tamoxifen.
Also, there were more
vascular deaths — 15 versus
seven. Is this a signal?
There is much interest in
this area. As the bone health
goes away, this surfaces—
but you could say these are
very small numbers, with
these relatively small differences,
when you’re in trials
involving thousands of patients.
Slide 18
 The Austrian Breast Cancer
Study Group 8 and ARNO
combined trials with women
on two years of tamoxifen.
These were all postmenopausal
women with earlystage
breast cancer, almost
all receptor-positive, who
were randomly assigned to
tamoxifen or anastrozole.
The Austrian Breast Cancer
Study Group 8 and ARNO
combined trials with women
on two years of tamoxifen.
These were all postmenopausal
women with earlystage
breast cancer, almost
all receptor-positive, who
were randomly assigned to
tamoxifen or anastrozole.
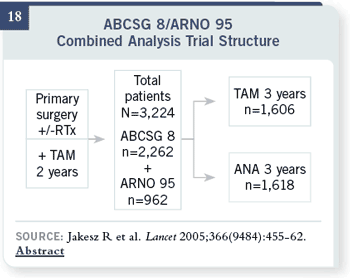
Slide 19
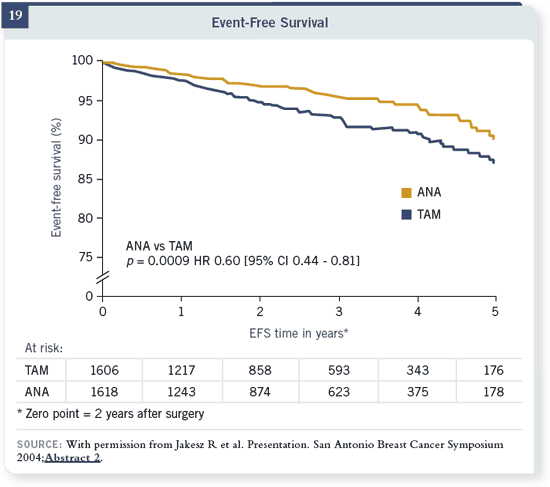
With that switching in the middle, at four standard deviations, there was a 40
percent reduction in recurrence risk. This trial of switching in the middle also
showed a trend towards a survival difference.
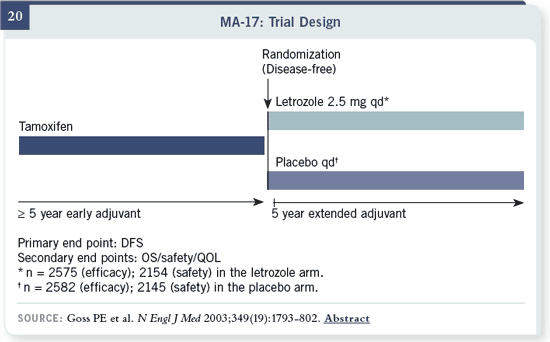
Slide 20
 There was a 42 percent
reduction in risk of recurrence
in the MA17 trial, with
switching at the end.
There was a 42 percent
reduction in risk of recurrence
in the MA17 trial, with
switching at the end.
Slide 21
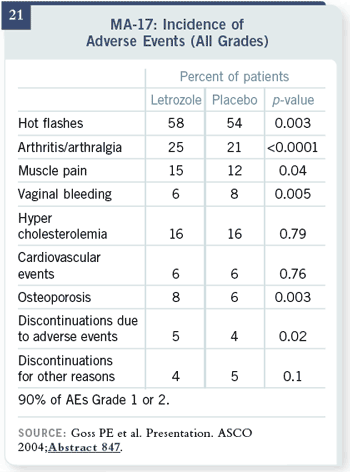
Look at the toxicity data. For
cardiovascular events, it’s six
percent with letrozole versus
six percent with placebo —
no difference. Hypercholesterolemia:
16 percent versus
16 percent — no difference.
What we have at the end is
this interesting dichotomy
between the BIG FEMTA
trial with Western European
patients, with randomization
at first, and the MA17 trial
with Western European, US
and UK patients having no
difference. That is an issue
that has not been completely
addressed.
Slide 22
 I think the bone health issue
is going away. At 33 months,
you can see numerically
more fractures on ATAC,
IES and MA17, but for all
these trials there was no prescreening
or ongoing therapy
for bones. One can calculate
that 70 percent of these
fractures occurred in women
who had preexisting osteoporosis
when they entered
the trial. You should
never get these numbers
if you’re doing screening
and intervention.
I think the bone health issue
is going away. At 33 months,
you can see numerically
more fractures on ATAC,
IES and MA17, but for all
these trials there was no prescreening
or ongoing therapy
for bones. One can calculate
that 70 percent of these
fractures occurred in women
who had preexisting osteoporosis
when they entered
the trial. You should
never get these numbers
if you’re doing screening
and intervention.
Slide 23
Just to show you that
bisphosphonates work — this
is ABCSG 12. Interestingly,
they take premenopausal
women with receptorpositive
disease, make
them postmenopausal with
goserelin acetate and then
give them anastrozole versus
tamoxifen. So these women
are made postmenopausal
very suddenly.
Slide 24
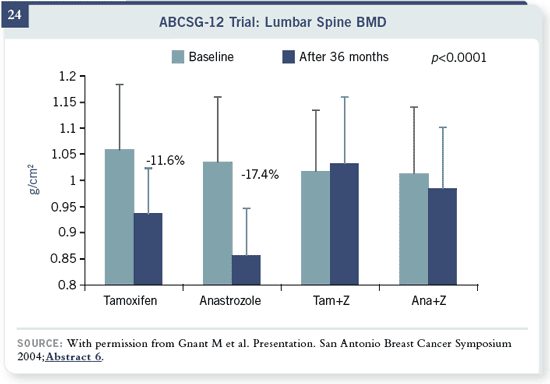
What happens when you
give them either zoledronic
acid or not? If you don’t
give them zoledronic acid,
you can see that tamoxifen
wasn’t able to fully prevent
this rapid loss of bone. There
was more loss with anastrozole,
which was completely
abrogated by zoledronic acid. Four milligrams of zoledronic acid every six
months completely abrogated bone loss. The Z-FAST trial had about the
same results.
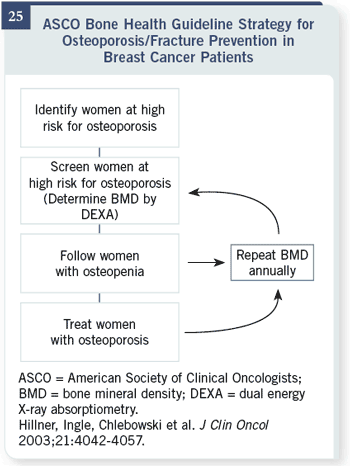
Slide 25
The ASCO bone health
guideline, which I helped
write, was published in
2003. We’re in the process of
updating it now, saying that
every woman should obtain
a bone mineral density
reading; that we should
follow or treat osteopenia as
an option, treat women with
osteoporosis and repeat bone
mineral density
tests annually.
We think that probably only
about 20 percent of women
then would need a bisphosphonate
while on aromatase
inhibitors, but we’ll have to
see if that’s the case or not.
Slide 26
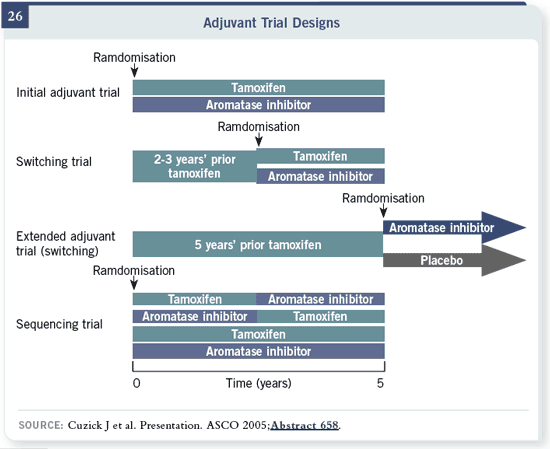
We’re going to finish by looking at the difference in these study designs. With
the ATAC and BIG FEMTA trials of tamoxifen versus aromatase inhibitors,
note that the randomization is at the start. With the switching trials, randomization
is after two or three years. That means the women who progressed
in the first two years, who had life-threatening toxicity of any kind, who
had a myocardial infarction or couldn’t take the pills aren’t here. So we don’t
know what the results would be if they randomized at the start. The only true
sequencing trial we have is BIG FEMTA, and its results are two or three years
away. You can see that the extended adjuvant trial is very much like
a switching trial, because you’re giving this and then the randomization
occurs. Women who relapse on tamoxifen, et cetera, won’t be around for the
second randomization.
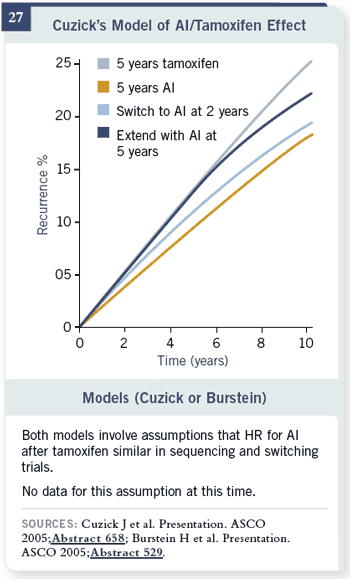
Slide 27
This effect has been alluded to in models. The problem that I have with the
models in terms of trying to figure out what’s going on is they all assume they
know what’s going to happen if you would have done the randomization at
first versus the randomization in the middle. I don’t think you can take those
numbers in the middle and tack them onto a model, because you just don’t know what happened in
the middle. The models are
interesting, but not
very informative.
Slide 28
In conclusion, these are the
rationales for using AIs:
Efficacy against early recurrence
peaks, side effects
defined in five years. If
you’re worried about the
long-term effects of an
aromatase inhibitor, I think
we’ve seen them, unless
you want to come up with
a hypothesis of why, after
estrogen comes back, you
expect to see further
side effects.
The side-effect profile is
favorable versus tamoxifen
for endometrial cancer,
stroke and PE. Bone loss is
preventable and treatable.
These are the rationales
for using tamoxifen: Long
experience with its use,
concern over side effects
with bone and coronary
heart disease with aromatase
inhibitors, no survival difference
in these studies and cost
associated with recurrence.
In the model analyses, all the
models say they know what
happens, and I don’t think
anybody does. Reasonable
oncologists can disagree.
* Presented at Research To Practice Breast Cancer Update CME Forum, Los Angeles, California,
May 21, 2005. The enclosed graphics from the presentation are included in the PowerPoint files on CD 3.
Please see page 1 for additional instructions.
SELECT PUBLICATIONS

|

