|

 DR SAAB: Ten years ago, at the age
of 58, this patient presented with a
right breast lump, which was breast
cancer. She was found to have diffuse
metastatic disease to the bones and was
in such pain that she needed radiation
therapy to various areas of the skeleton
over the subsequent 18 months. The
tumor was hormone receptor-positive,
and her prior oncologist started her
on tamoxifen. Over the ensuing two
years, she didn’t respond very well, was
found to be resistant to tamoxifen and
needed a substantial amount of radiation
therapy. She was switched to anastrozole,
which again was determined not
to be effective. DR SAAB: Ten years ago, at the age
of 58, this patient presented with a
right breast lump, which was breast
cancer. She was found to have diffuse
metastatic disease to the bones and was
in such pain that she needed radiation
therapy to various areas of the skeleton
over the subsequent 18 months. The
tumor was hormone receptor-positive,
and her prior oncologist started her
on tamoxifen. Over the ensuing two
years, she didn’t respond very well, was
found to be resistant to tamoxifen and
needed a substantial amount of radiation
therapy. She was switched to anastrozole,
which again was determined not
to be effective.
Eventually, she was offered chemotherapy,
which she had declined
initially. She was given CMF with
filgrastim support over a period of six
months. Toward the end of 1999, again
because of rising tumor markers, it
was determined that the chemotherapy
wasn’t working. Her oncologist then
tried low-dose paclitaxel every week for
three months, and he determined that it
didn’t work well.
This lady required morphine all this
time, despite radiation therapy. In
January 2000, she was started on
capecitabine, and she has been on it
ever since. For a full five years on
capecitabine, she had been morphine-free
and pain-free, until three months
ago, when her tumor markers started
rising again and her pain started to
develop in different areas. Obviously,
her cancer was coming back. I then
started her on docetaxel every three
weeks. After the second cycle, her
tumor markers went down and she
experienced symptom improvement.
 DR OSBORNE: When she experienced
progression of disease on the hormone
therapy, how long had she been on it,
and was it more than just markers going
up that led to the discontinuation? DR OSBORNE: When she experienced
progression of disease on the hormone
therapy, how long had she been on it,
and was it more than just markers going
up that led to the discontinuation?
 DR SAAB: Her symptoms, including
pain, persisted, and her markers
continued to rise. The CA27.29 and
CA15-3 were elevated around 150 to
180 and her CEA was a little higher. DR SAAB: Her symptoms, including
pain, persisted, and her markers
continued to rise. The CA27.29 and
CA15-3 were elevated around 150 to
180 and her CEA was a little higher.
 DR GRADISHAR: This patient has had
a remarkable course on capecitabine.
Capecitabine is one of my favorite
chemotherapy drugs. I believe it was
probably underrated initially until we
learned how to properly dose it and
utilize it effectively in both older and
younger patients. I think one of the
issues is whether combining drugs
is better than using single agents in
sequence and how you actually define
superiority. DR GRADISHAR: This patient has had
a remarkable course on capecitabine.
Capecitabine is one of my favorite
chemotherapy drugs. I believe it was
probably underrated initially until we
learned how to properly dose it and
utilize it effectively in both older and
younger patients. I think one of the
issues is whether combining drugs
is better than using single agents in
sequence and how you actually define
superiority.
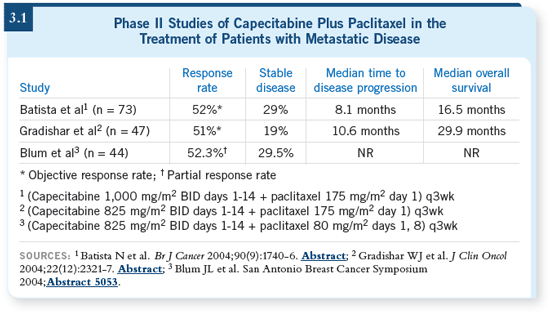
One experience, presented by Joanne
Blum, evaluated capecitabine plus a
weekly schedule of paclitaxel (Blum
2004). When you compare the clinical
endpoints in that trial to the every
three-week schedule of paclitaxel in
both the European and US studies, you
see a response rate in the 50 percent range (3.1). In terms of adverse events,
they’re modest, with the most prominent
one being hand-foot syndrome,
and then hematologic toxicities, which
were fairly predictable.
 DR LOVE: Peter, we have found that
clinical research leaders much more
commonly use capecitabine than clinicians
in practice. What has been your
experience with capecitabine? DR LOVE: Peter, we have found that
clinical research leaders much more
commonly use capecitabine than clinicians
in practice. What has been your
experience with capecitabine?
 DR RAVDIN: Capecitabine has some
attractive features. I view it, in terms
of toxicity and response, as something
that bridges the gap between hormonal
therapy and intravenous chemotherapy.
Particularly when dosed a bit lower
than the package insert dose, it’s tolerable
for most patients, and they don’t
experience nausea, vomiting or hair
loss, almost as if they were receiving an
endocrine agent. It’s an oral agent, we
don’t have to put in a line, so it’s easier
for patients to accept. I think all those
features make it an attractive agent. DR RAVDIN: Capecitabine has some
attractive features. I view it, in terms
of toxicity and response, as something
that bridges the gap between hormonal
therapy and intravenous chemotherapy.
Particularly when dosed a bit lower
than the package insert dose, it’s tolerable
for most patients, and they don’t
experience nausea, vomiting or hair
loss, almost as if they were receiving an
endocrine agent. It’s an oral agent, we
don’t have to put in a line, so it’s easier
for patients to accept. I think all those
features make it an attractive agent.
Actually, I’m a little surprised that
it isn’t more commonly used in the
community, because I think it’s one of
those agents that is generally tolerated
with repeated use. With a lot of other
agents, patients begin to get tired when
you get in six cycles.
 DR LOVE: Aman, what was your take
on the bevacizumab/paclitaxel data
initially presented by Kathy Miller at
ASCO (Miller 2005a)? DR LOVE: Aman, what was your take
on the bevacizumab/paclitaxel data
initially presented by Kathy Miller at
ASCO (Miller 2005a)?
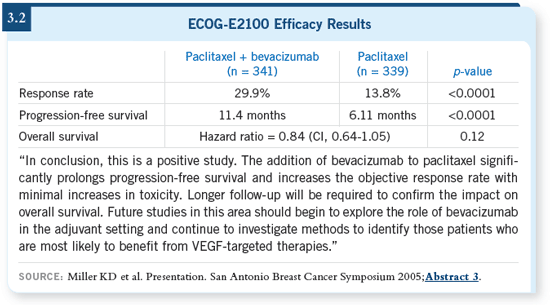
 DR BUZDAR: I think if you put it in
context, it’s very similar to the data we
saw when trastuzumab was combined in
the first-line setting: There is a significantly
higher response rate, longer
control of disease and early evidence
that it may have a favorable impact on
survival (3.2). Its side-effect profile is
unique, but I think most of the side
effects are very manageable, and no new
side effects were seen in this study. DR BUZDAR: I think if you put it in
context, it’s very similar to the data we
saw when trastuzumab was combined in
the first-line setting: There is a significantly
higher response rate, longer
control of disease and early evidence
that it may have a favorable impact on
survival (3.2). Its side-effect profile is
unique, but I think most of the side
effects are very manageable, and no new
side effects were seen in this study.
With regard to this specific patient,
would I consider adding a biologic
agent to her therapy? The data on
capecitabine combined with bevacizumab,
unfortunately, did not show
a longer time to progression or any
favorable impact on survival, although a
higher number of patients had an objective
response to the therapy (Miller
2005b). So I think these therapies may
need to be utilized early on to get
the benefit.
 DR LOVE: Are you using bevacizumab
and paclitaxel in the first-line setting? DR LOVE: Are you using bevacizumab
and paclitaxel in the first-line setting?
 DR BUZDAR: I think the data are very
compelling, and I feel that it is our
responsibility to share that information
with every patient who has similar
eligibility criteria as those in that
ECOG study. DR BUZDAR: I think the data are very
compelling, and I feel that it is our
responsibility to share that information
with every patient who has similar
eligibility criteria as those in that
ECOG study.
 DR LOVE: Kent, what are your
thoughts on this? DR LOVE: Kent, what are your
thoughts on this?
 DR OSBORNE: I certainly wouldn’t use
bevacizumab in any situation other than
first-line therapy, since that’s where the
data came from, so I wouldn’t use it in
this patient. But I’m very concerned
about the cost of treatment for a few
extra months in time to progression. If
I had insurance that paid 80 percent,
I’m not sure I would elect to receive
bevacizumab because 20 percent of a
lot of money is a lot of money. I’m very
concerned about the cost issue. DR OSBORNE: I certainly wouldn’t use
bevacizumab in any situation other than
first-line therapy, since that’s where the
data came from, so I wouldn’t use it in
this patient. But I’m very concerned
about the cost of treatment for a few
extra months in time to progression. If
I had insurance that paid 80 percent,
I’m not sure I would elect to receive
bevacizumab because 20 percent of a
lot of money is a lot of money. I’m very
concerned about the cost issue.
 DR LOVE: Peter, do you agree with
Kent that you would only use it in that
specific situation? DR LOVE: Peter, do you agree with
Kent that you would only use it in that
specific situation?
Our group has been tracking the
bevacizumab story in colorectal cancer.
The initial data were presented two
years ago with IFL, and yet everybody
immediately started using it with
FOLFOX, and eventually that combination
was shown to be effective. What
are your thoughts about using bevacizumab
in breast cancer, and what agents
would you combine it with?
 DR RAVDIN: I largely agree with
what Kent has said in that while a lot
of expense, and even sometimes a lot
of toxicity, is justified in the adjuvant
arena, in the metastatic disease arena,
enormous expense is sometimes added
to the cost of therapy for a relatively
small impact. Data from one trial that’s
not a crossover trial are not strong
enough to make me consider it the
standard of care as front-line therapy
for everyone with hormone refractory,
metastatic breast cancer because I think
that would actually break the back of
the healthcare system. DR RAVDIN: I largely agree with
what Kent has said in that while a lot
of expense, and even sometimes a lot
of toxicity, is justified in the adjuvant
arena, in the metastatic disease arena,
enormous expense is sometimes added
to the cost of therapy for a relatively
small impact. Data from one trial that’s
not a crossover trial are not strong
enough to make me consider it the
standard of care as front-line therapy
for everyone with hormone refractory,
metastatic breast cancer because I think
that would actually break the back of
the healthcare system.
Select publications
|

