|
 DR DRULLINSKY: This patient first
presented in 1989 with an infiltrating
lobular carcinoma and 21 positive
lymph nodes. She was treated at a local
hospital with CMF and etoposide in the
adjuvant setting and placed on tamoxifen
for five years. DR DRULLINSKY: This patient first
presented in 1989 with an infiltrating
lobular carcinoma and 21 positive
lymph nodes. She was treated at a local
hospital with CMF and etoposide in the
adjuvant setting and placed on tamoxifen
for five years.
Then she was watched for several
months and developed bone metastases.
At this point, she was switched
to an aromatase inhibitor, anastrozole.
Then she quickly developed aggressive
disease, mostly extending to the bones,
and she had mild ascites.
At this point, 11 years after presentation,
she has been through all the
hormones, all the aromatase inhibitors,
and fulvestrant. She has had a number
of chemotherapy agents, including
capecitabine, gemcitabine, vinorelbine,
and every three-week docetaxel.
At the end of last year, she developed
extensive bone marrow involvement,
complicated by anemia and thrombocytopenia
requiring transfusions. Her
baseline platelet count last year was
nine, and her hemoglobin was five.
The only treatment she hadn’t received
was an anthracycline. So I gave her
liposomal doxorubicin, and she had a
one-year response. She became transfusion-
free from both red blood cells
and platelets. Now I’m faced with the
fact that she’s progressed on liposomal
doxorubicin, and she’s again thrombocytopenic,
anemic and dependent on
transfusions.
 DR LOVE: What about her symptomatology
and performance status? DR LOVE: What about her symptomatology
and performance status?
 DR DRULLINSKY: She is completely
asymptomatic. She does not take any
narcotics at all, and her performance
status is 90 percent. DR DRULLINSKY: She is completely
asymptomatic. She does not take any
narcotics at all, and her performance
status is 90 percent.
 DR LOVE: Has she experienced any
bleeding? DR LOVE: Has she experienced any
bleeding?
 DR DRULLINSKY: No. DR DRULLINSKY: No.
 DR LOVE: Which taxanes has she
received? DR LOVE: Which taxanes has she
received?
 DR DRULLINSKY: She’s only had every
three-week docetaxel. And with that,
she actually had stable disease. DR DRULLINSKY: She’s only had every
three-week docetaxel. And with that,
she actually had stable disease.
 DR LOVE: What are you thinking
about at this point? DR LOVE: What are you thinking
about at this point?
 DR DRULLINSKY: Well, she has not
yet received the platinums and weekly
taxanes. Given the data with bevacizumab,
I was considering that. The
problem is she’s very thrombocytopenic,
with a platelet count under 10. DR DRULLINSKY: Well, she has not
yet received the platinums and weekly
taxanes. Given the data with bevacizumab,
I was considering that. The
problem is she’s very thrombocytopenic,
with a platelet count under 10.
 DR LOVE: Aman, any thoughts about
her case? DR LOVE: Aman, any thoughts about
her case?
 DR BUZDAR: One thing you may
consider is weekly paclitaxel or nab paclitaxel. With weekly paclitaxel,
myelosuppression is not one of the side
effects. She may benefit, even though she has been treated with docetaxel.
There is a partial lack of cross-resistance
between these two agents, and the lack
of myelotoxicity might be worthwhile.
The same is true with nab paclitaxel,
which may be another option for you
to consider. DR BUZDAR: One thing you may
consider is weekly paclitaxel or nab paclitaxel. With weekly paclitaxel,
myelosuppression is not one of the side
effects. She may benefit, even though she has been treated with docetaxel.
There is a partial lack of cross-resistance
between these two agents, and the lack
of myelotoxicity might be worthwhile.
The same is true with nab paclitaxel,
which may be another option for you
to consider.
 DR LOVE: You mentioned that she had
a good response to liposomal doxorubicin.
Along the way, as she’s gotten
these various therapies, is there anything
else that she had a good response to? DR LOVE: You mentioned that she had
a good response to liposomal doxorubicin.
Along the way, as she’s gotten
these various therapies, is there anything
else that she had a good response to?
 DR DRULLINSKY: Every therapy she
has had has worked. DR DRULLINSKY: Every therapy she
has had has worked.
 DR LOVE: How did she do on
capecitabine? DR LOVE: How did she do on
capecitabine?
 DR DRULLINSKY: Capecitabine worked
for one year. Every treatment regimen
worked for approximately nine months
to one year. DR DRULLINSKY: Capecitabine worked
for one year. Every treatment regimen
worked for approximately nine months
to one year.
 DR LOVE: John, what are your
thoughts? DR LOVE: John, what are your
thoughts?
 DR MACKEY: Perhaps this is heresy,
but at some point you have to have a
conversation with the patient about
what realistically she can expect from
further chemotherapy. You could kill
this woman with your next cycle,
potentially. She has to be aware of that.
In our center in Edmonton, we have
a world-class palliative care team.
At this point, we would hand over
this woman’s care to the palliative
care team. DR MACKEY: Perhaps this is heresy,
but at some point you have to have a
conversation with the patient about
what realistically she can expect from
further chemotherapy. You could kill
this woman with your next cycle,
potentially. She has to be aware of that.
In our center in Edmonton, we have
a world-class palliative care team.
At this point, we would hand over
this woman’s care to the palliative
care team.
 DR SEIDMAN: Having just completed
a two-week stint as the inpatient
attending at Memorial Sloan-Kettering,
I wish I were in Canada right now
with Dr Mackey. Obviously, no further
chemotherapy and best supportive care
certainly should be part of a discussion
with someone when they’re facing
their sixth line of therapy. Despite
the dangerously low platelet count
that Pam described, I did get a sense
that this patient has a high functional
status and is living a good quality of
life. She has repeatedly had at least
transient responses to every chemotherapy
regimen she’s been exposed to.
I think an overall difference in orientation
exists toward breast cancer and
the utility and futility of chemotherapy
between Canada and the US. DR SEIDMAN: Having just completed
a two-week stint as the inpatient
attending at Memorial Sloan-Kettering,
I wish I were in Canada right now
with Dr Mackey. Obviously, no further
chemotherapy and best supportive care
certainly should be part of a discussion
with someone when they’re facing
their sixth line of therapy. Despite
the dangerously low platelet count
that Pam described, I did get a sense
that this patient has a high functional
status and is living a good quality of
life. She has repeatedly had at least
transient responses to every chemotherapy
regimen she’s been exposed to.
I think an overall difference in orientation
exists toward breast cancer and
the utility and futility of chemotherapy
between Canada and the US.
 DR LOVE: What, specifically, would
you recommend, Andy? DR LOVE: What, specifically, would
you recommend, Andy?
 DR SEIDMAN: I would include in a
discussion the possibility that chemotherapy
could lead to life-threatening
hemorrhagic complications. If
the patient still wanted to proceed,
the regimens that would likely not
contribute further to thrombocytopenia
— weekly paclitaxel or weekly nab paclitaxel — are reasonable options.
Other drugs I would consider, if she
had a healthier bone marrow, include
agents like irinotecan, for which we
have Phase II data in the anthracycline/
taxane-refractory population (Perez
2004). Even agents such as pemetrexed,
for which there are Phase II data
(O’Shaughnessy 2005). DR SEIDMAN: I would include in a
discussion the possibility that chemotherapy
could lead to life-threatening
hemorrhagic complications. If
the patient still wanted to proceed,
the regimens that would likely not
contribute further to thrombocytopenia
— weekly paclitaxel or weekly nab paclitaxel — are reasonable options.
Other drugs I would consider, if she
had a healthier bone marrow, include
agents like irinotecan, for which we
have Phase II data in the anthracycline/
taxane-refractory population (Perez
2004). Even agents such as pemetrexed,
for which there are Phase II data
(O’Shaughnessy 2005).
 DR RAVDIN: I would largely agree with
what has already been said. I think that
weekly taxane therapy would be my
first pick in this patient. The one thing
I would also keep in the back of my
mind is the possibility, when her platelets
are back at 100,000, of rebiopsying
her tumor. There are times, particularly
over long periods, where the biology
of the tumor can drift. Maybe she’s
actually HER2-positive at this point. DR RAVDIN: I would largely agree with
what has already been said. I think that
weekly taxane therapy would be my
first pick in this patient. The one thing
I would also keep in the back of my
mind is the possibility, when her platelets
are back at 100,000, of rebiopsying
her tumor. There are times, particularly
over long periods, where the biology
of the tumor can drift. Maybe she’s
actually HER2-positive at this point.
 DR DRULLINSKY: I do have the bone
marrow from a year and a half ago, and
it was completely packed, so maybe we
could test that. DR DRULLINSKY: I do have the bone
marrow from a year and a half ago, and
it was completely packed, so maybe we
could test that.
 DR LOVE: Do we know what the
incidence is of switching from HER2-
negative to HER2-positive, or an
original false negative that you would
now identify as positive? Andy? DR LOVE: Do we know what the
incidence is of switching from HER2-
negative to HER2-positive, or an
original false negative that you would
now identify as positive? Andy?
 DR SEIDMAN: Lindsey Harris and
others have reported on this. Dr Luftner
from Germany recently reported that
the incidence is in the range of 10 to
15 percent discordance (Luftner 2004).
More commonly, the direction tends
to be negative to positive, rather than
positive to negative. This probably
does warrant rebiopsying patients,
specifically when you’re running out
of options and when a biopsy doesn’t
represent a major ordeal for the patient. DR SEIDMAN: Lindsey Harris and
others have reported on this. Dr Luftner
from Germany recently reported that
the incidence is in the range of 10 to
15 percent discordance (Luftner 2004).
More commonly, the direction tends
to be negative to positive, rather than
positive to negative. This probably
does warrant rebiopsying patients,
specifically when you’re running out
of options and when a biopsy doesn’t
represent a major ordeal for the patient.
 DR LOVE: Let’s talk about the typical
case of a patient who doesn’t have
rapidly progressive, visceral, symptomatic
metastatic disease that requires
an immediate response — the patient
who’s had AC/paclitaxel, a very
common adjuvant regimen in patients
who relapse. Starting with John, in
patients with ER-negative, HER2-
negative disease, how have you thought
through the decision at that point?
And did your thought process change
after you saw the bevacizumab data
(Miller 2005a)? DR LOVE: Let’s talk about the typical
case of a patient who doesn’t have
rapidly progressive, visceral, symptomatic
metastatic disease that requires
an immediate response — the patient
who’s had AC/paclitaxel, a very
common adjuvant regimen in patients
who relapse. Starting with John, in
patients with ER-negative, HER2-
negative disease, how have you thought
through the decision at that point?
And did your thought process change
after you saw the bevacizumab data
(Miller 2005a)?
 DR MACKEY: The reality is, we have a
lot of patients treated with an anthracycline
and a taxane in the adjuvant
setting who relapse. At the time of
relapse, it’s always nice to be able to
offer them an option where they don’t
lose their hair and they can have outpatient
oral therapy. So we’ve made a
major shift to first-line capecitabine.
It’s a well-tolerated, often very effective
option. Unlike the data we’re
getting from the vinorelbine and the
gemcitabine studies, some of these
patients have a tremendous prolonged
response. I’m not saying the median
times to progression are substantially
greater, but we are seeing at two
and a half and three and four years
people who are still on at cycle 70 of
capecitabine. DR MACKEY: The reality is, we have a
lot of patients treated with an anthracycline
and a taxane in the adjuvant
setting who relapse. At the time of
relapse, it’s always nice to be able to
offer them an option where they don’t
lose their hair and they can have outpatient
oral therapy. So we’ve made a
major shift to first-line capecitabine.
It’s a well-tolerated, often very effective
option. Unlike the data we’re
getting from the vinorelbine and the
gemcitabine studies, some of these
patients have a tremendous prolonged
response. I’m not saying the median
times to progression are substantially
greater, but we are seeing at two
and a half and three and four years
people who are still on at cycle 70 of
capecitabine.
 DR LOVE: We know there are
reimbursement and cost issues that enter
into this, but looking at the pure science
in terms of decision-making, John,
what about bevacizumab? Would you
combine it with capecitabine? Where,
right now, would you utilize it in the
metastatic setting? DR LOVE: We know there are
reimbursement and cost issues that enter
into this, but looking at the pure science
in terms of decision-making, John,
what about bevacizumab? Would you
combine it with capecitabine? Where,
right now, would you utilize it in the
metastatic setting?
 DR MACKEY: There are two ways to
look at the bevacizumab data. What
makes it particularly exciting is that
it’s a new therapeutic target. We’ve
introduced antiangiogenic therapy.
But if you look at ways of beating
single-agent paclitaxel at 175 mg/m2
every three weeks, bevacizumab is
number six on the list. The excitement
is because of the possibility of a
new mechanism, almost as much as the
improved progression-free and overall
survival (Miller 2005a). Until such
time as we have bevacizumab available
for breast cancer treatment in Canada,
which it currently is not, we are stuck
with looking at what provides optimal
survival with the agents that we have.
I apologize. We have a pragmatic
barrier here. DR MACKEY: There are two ways to
look at the bevacizumab data. What
makes it particularly exciting is that
it’s a new therapeutic target. We’ve
introduced antiangiogenic therapy.
But if you look at ways of beating
single-agent paclitaxel at 175 mg/m2
every three weeks, bevacizumab is
number six on the list. The excitement
is because of the possibility of a
new mechanism, almost as much as the
improved progression-free and overall
survival (Miller 2005a). Until such
time as we have bevacizumab available
for breast cancer treatment in Canada,
which it currently is not, we are stuck
with looking at what provides optimal
survival with the agents that we have.
I apologize. We have a pragmatic
barrier here.
 DR LOVE: Andy, how do you approach
patients like this, and where does
bevacizumab fit in? DR LOVE: Andy, how do you approach
patients like this, and where does
bevacizumab fit in?
 DR SEIDMAN: Clearly, there are
patients who have minimal and
sometimes no symptoms of disease
for whom hormones are just not the
right choice. Their disease is ER/PR-negative,
or you’ve exhausted all of your
reasonable hormonal options. In an
effort not to make the treatment worse
than the disease, oral capecitabine is a
very attractive option. Patients live their
lives at home, not in your oncology
clinic. DR SEIDMAN: Clearly, there are
patients who have minimal and
sometimes no symptoms of disease
for whom hormones are just not the
right choice. Their disease is ER/PR-negative,
or you’ve exhausted all of your
reasonable hormonal options. In an
effort not to make the treatment worse
than the disease, oral capecitabine is a
very attractive option. Patients live their
lives at home, not in your oncology
clinic.
We will have some data on this from
a Mexican oncology group trial that
was unfortunately presented way too
prematurely at ASCO within the last
couple of years (Soto 2003). This was
a randomized study of capecitabine first, followed by taxane, a taxane first
followed by capecitabine, or the combination
of taxane and capecitabine. With
mature results from that trial, maybe
we’ll be more reassured that using
capecitabine as monotherapy, as chapter
one, is perfectly appropriate.
 DR LOVE: Andy, you’re saying that in
patients who’ve had prior dose-dense
ACT, in general, you’re going to start
with capecitabine. Is bevacizumab going
to work its way into the algorithm of a
patient like that? DR LOVE: Andy, you’re saying that in
patients who’ve had prior dose-dense
ACT, in general, you’re going to start
with capecitabine. Is bevacizumab going
to work its way into the algorithm of a
patient like that?
 DR SEIDMAN: When I think about
what to use after adjuvant anthracycline/
taxane-based therapy, the thing
I think mostly about is how long it
has been since they’ve completed that
therapy. DR SEIDMAN: When I think about
what to use after adjuvant anthracycline/
taxane-based therapy, the thing
I think mostly about is how long it
has been since they’ve completed that
therapy.
If it’s been a relatively short period of
time — there’s nothing magic about
one year, but we often use that as a
benchmark — I’m not that enthusiastic
about going back and using
a taxane or an anthracycline again.
At that particular moment in time,
capecitabine is an obvious default
option. Given the increased response
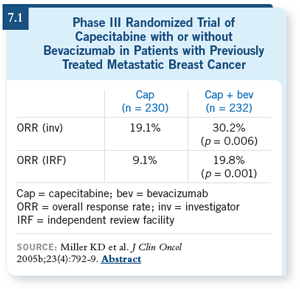
rate seen with bevacizumab when added
to capecitabine (Miller 2005b; [7.1]),
as long as your insurance companies
will recognize the benefits seen with
this drug in combination with other
agents, and in other diseases, and in
combination with paclitaxel in the
first-line setting for metastatic disease, it
definitely seems to be a rational option.
 DR LOVE: With bevacizumab, the cost
and reimbursement issues obviously are
out on the table. We don’t know how
that’s going to play out. We’re focused
on the science today, but I think,
obviously, that’s a huge issue. Aman,
same question: Patient has had prior
AC/paclitaxel, asymptomatic metastatic
disease. What’s your algorithm? And is
bevacizumab going to fit into it? DR LOVE: With bevacizumab, the cost
and reimbursement issues obviously are
out on the table. We don’t know how
that’s going to play out. We’re focused
on the science today, but I think,
obviously, that’s a huge issue. Aman,
same question: Patient has had prior
AC/paclitaxel, asymptomatic metastatic
disease. What’s your algorithm? And is
bevacizumab going to fit into it?
 DR BUZDAR: I discuss the data, which
were presented by Kathy Miller, with
these types of patients. I think the point
that Andy made is very important:
If the patient failed very shortly after
receiving the taxane in the adjuvant
setting, then I think capecitabine might
be a better option, and the taxane might
not be the appropriate option. But if
the patient has a longer disease-free
interval, I think we need to discuss that
because of two points. There is substantial
improvement in time to progression
and early evidence to suggest that
bevacizumab may have a favorable
impact on survival (Miller 2005a). DR BUZDAR: I discuss the data, which
were presented by Kathy Miller, with
these types of patients. I think the point
that Andy made is very important:
If the patient failed very shortly after
receiving the taxane in the adjuvant
setting, then I think capecitabine might
be a better option, and the taxane might
not be the appropriate option. But if
the patient has a longer disease-free
interval, I think we need to discuss that
because of two points. There is substantial
improvement in time to progression
and early evidence to suggest that
bevacizumab may have a favorable
impact on survival (Miller 2005a).
 DR LOVE: Again, cost aside, Aman,
would you bring up the issue of bevacizumab
plus capecitabine? DR LOVE: Again, cost aside, Aman,
would you bring up the issue of bevacizumab
plus capecitabine?
 DR BUZDAR: With bevacizumab and
capecitabine, I think the data, which
were in a somewhat more heavily
treated patient population, unfortunately,
did not pan out into longer
control of disease or any favorable
impact on the survival. Although
investigator-reported data and independently
peer-reviewed data did show that
women who received the combination
of bevacizumab and capecitabine had
a higher objective regression of their
disease, it somehow did not translate
into a time to progression or survival
benefit (Miller 2005b; [7.1]). DR BUZDAR: With bevacizumab and
capecitabine, I think the data, which
were in a somewhat more heavily
treated patient population, unfortunately,
did not pan out into longer
control of disease or any favorable
impact on the survival. Although
investigator-reported data and independently
peer-reviewed data did show that
women who received the combination
of bevacizumab and capecitabine had
a higher objective regression of their
disease, it somehow did not translate
into a time to progression or survival
benefit (Miller 2005b; [7.1]).
 DR LOVE: Peter, how do you approach
the choice of chemotherapy in a patient
who received prior AC/paclitaxel? DR LOVE: Peter, how do you approach
the choice of chemotherapy in a patient
who received prior AC/paclitaxel?
 DR RAVDIN: Actually, capecitabine
is my first choice because of all the
reasons that have already been stated:
low toxicity and the sometimes very
long duration of responses. One thing
I’ve been doing more is thinking about
new agents in Phase I testing very early
in those patients who don’t have threatening
disease. I always used to give
these as fifth- and sixth-line therapy, but by then, eligibility issues
come up: central nervous
system metastases, bone
marrow problems. DR RAVDIN: Actually, capecitabine
is my first choice because of all the
reasons that have already been stated:
low toxicity and the sometimes very
long duration of responses. One thing
I’ve been doing more is thinking about
new agents in Phase I testing very early
in those patients who don’t have threatening
disease. I always used to give
these as fifth- and sixth-line therapy, but by then, eligibility issues
come up: central nervous
system metastases, bone
marrow problems.
I’ve recently become aware
of how many people have
been disappointed in the idea
that they wanted to receive
something new, but it was only
thought of when they were
so far down the road and had
other comorbid problems that
they were no longer eligible.
So I often tell people who are
two or three treatments in: If
we’re going to try a wild card,
now is the time to try it, when
you’re in relatively good shape;
let’s not wait until your eligibility
might be in question.
Select publications
|

