|

 DR RAPPAPORT: This is a 41-year-old
woman who previously had a hysterectomy
and bilateral oophorectomy for
menometrorrhagia four years ago at
the age of 37. She underwent a radical
mastectomy for a 3.5-centimeter,
poorly differentiated adenocarcinoma,
metastatic to two lymph nodes. DR RAPPAPORT: This is a 41-year-old
woman who previously had a hysterectomy
and bilateral oophorectomy for
menometrorrhagia four years ago at
the age of 37. She underwent a radical
mastectomy for a 3.5-centimeter,
poorly differentiated adenocarcinoma,
metastatic to two lymph nodes.
She was treated with FAC times six,
and currently is on tamoxifen, which
she has taken for three and a half years.
The tumor is both ER- and PR-positive
and overexpresses HER2 by FISH.
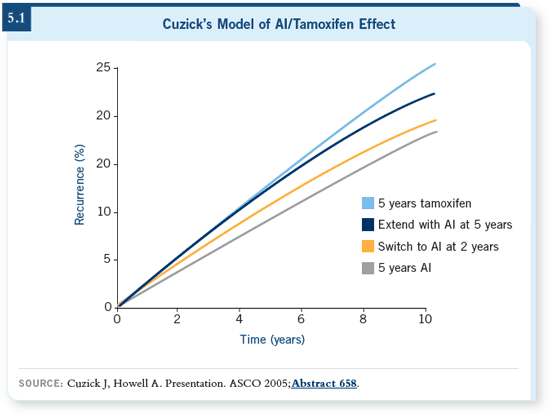
 DR LOVE: This case raises the issue as
to the optimal long-term endocrine
treatment strategy in postmenopausal
women. A number of theoretical
models have been proposed to estimate
whether an aromatase inhibitor initially
or tamoxifen followed by an aromatase
inhibitor is the optimal schedule (5.1).
Dr Chlebowski, could you comment on
these models? DR LOVE: This case raises the issue as
to the optimal long-term endocrine
treatment strategy in postmenopausal
women. A number of theoretical
models have been proposed to estimate
whether an aromatase inhibitor initially
or tamoxifen followed by an aromatase
inhibitor is the optimal schedule (5.1).
Dr Chlebowski, could you comment on
these models?
 DR CHLEBOWSKI: The problem I have
with the models is they all assume they
know what’s going to happen if you
randomly assigned patients at first versus
in the middle. I don’t think you can
take those numbers in the middle and
tack them onto a model. The models
are interesting but not very informative. DR CHLEBOWSKI: The problem I have
with the models is they all assume they
know what’s going to happen if you
randomly assigned patients at first versus
in the middle. I don’t think you can
take those numbers in the middle and
tack them onto a model. The models
are interesting but not very informative.
The side-effect profile is more favorable
for aromatase inhibitors than tamoxifen
for endometrial cancer, stroke and PE.
Bone loss is preventable and treatable.
 DR LOVE: Can you discuss the
trial reported at ASCO 2005 evaluating
anastrozole in patients who had
completed five years of tamoxifen? DR LOVE: Can you discuss the
trial reported at ASCO 2005 evaluating
anastrozole in patients who had
completed five years of tamoxifen?
 DR CHLEBOWSKI: The Austrian trial,
ABCSG-6a, looked at patients who had
received tamoxifen with or without
aminoglutethimide for five years. The
patients were then randomly assigned
to anastrozole or no treatment for three
years. This extended adjuvant trial also
showed a benefit from anastrozole. DR CHLEBOWSKI: The Austrian trial,
ABCSG-6a, looked at patients who had
received tamoxifen with or without
aminoglutethimide for five years. The
patients were then randomly assigned
to anastrozole or no treatment for three
years. This extended adjuvant trial also
showed a benefit from anastrozole.
 DR LOVE: How did those data compare
to what was seen in the MA17 trial
with letrozole? DR LOVE: How did those data compare
to what was seen in the MA17 trial
with letrozole?
 DR CHLEBOWSKI: The data looked
about the same. Of course, in the letrozole
trial, we talk about five years of
letrozole, but it was reported after two
and a half years of treatment. DR CHLEBOWSKI: The data looked
about the same. Of course, in the letrozole
trial, we talk about five years of
letrozole, but it was reported after two
and a half years of treatment.
 DR LOVE: What you were saying about
the models makes sense in terms of the
point of randomization and the fact that
looking at how relapse rate is affected
at two or five years does not take into
account all the recurrences and adverse
events that occurred before that time. DR LOVE: What you were saying about
the models makes sense in terms of the
point of randomization and the fact that
looking at how relapse rate is affected
at two or five years does not take into
account all the recurrences and adverse
events that occurred before that time.
 DR CHLEBOWSKI: To me, that’s a big
black box, to make that assumption.
The other part of it is, when you look at
the assumptions, trying to compare Jack
Cuzick’s and Hal Burstein’s model, they
get completely different answers. DR CHLEBOWSKI: To me, that’s a big
black box, to make that assumption.
The other part of it is, when you look at
the assumptions, trying to compare Jack
Cuzick’s and Hal Burstein’s model, they
get completely different answers.
Burstein used the primary studies’
main endpoints and Cuzick used a little
different endpoint, and you get a totally
different result, based on what you put
in the model.
 DR LOVE: Dan, from a clinical point
of view, the idea of starting tamoxifen
and switching to an AI requires clinicians
to tell patients, “Here’s my long-term
strategy for you. You’re going to
go on a therapy that, for the first two or
three years, is going to expose you to a
greater risk of relapse, but in the long
run, you’ll have fewer relapses.” DR LOVE: Dan, from a clinical point
of view, the idea of starting tamoxifen
and switching to an AI requires clinicians
to tell patients, “Here’s my long-term
strategy for you. You’re going to
go on a therapy that, for the first two or
three years, is going to expose you to a
greater risk of relapse, but in the long
run, you’ll have fewer relapses.”
 DR HAYES: In my own practice, I
believe that for patients who have a
reasonably good prognosis, tamoxifen
alone is a perfectly adequate start. I
actually disagree a bit with Rowan in
that I’m not sure we know about long-term
follow-up of women who have
complete estrogen depletion. It took us
a long time to see some of the unanticipated
effects of tamoxifen. DR HAYES: In my own practice, I
believe that for patients who have a
reasonably good prognosis, tamoxifen
alone is a perfectly adequate start. I
actually disagree a bit with Rowan in
that I’m not sure we know about long-term
follow-up of women who have
complete estrogen depletion. It took us
a long time to see some of the unanticipated
effects of tamoxifen.
Tamoxifen is a good drug. It’s not like
we should throw it out. In patients who
start out with a good prognosis, the
maximum potential added benefit of an
AI over tamoxifen can’t be more than
one or two percent absolute survival
over 10 years or so. Given what we
know about tamoxifen and the benefits
of it, I have started with that.
On the other hand, in this patient with
positive nodes, I would consider starting
with an aromatase inhibitor initially.
She has a high risk of relapse up front,
so the potential added proportional
reduction in recurrences and death by
using an AI is amplified. In addition, if
you want to bring Kent’s biology into
it, she’s HER2-positive, and one would
believe that estrogen depletion should
be a more effective strategy than a
SERM in such a patient.
 DR LOVE: Would you describe the
type of patient you would treat with
tamoxifen up front and discuss the issue
of excess toxicities, such as endometrial
cancer and stroke? DR LOVE: Would you describe the
type of patient you would treat with
tamoxifen up front and discuss the issue
of excess toxicities, such as endometrial
cancer and stroke?
 DR HAYES: First of all, let’s be clear
that we’re talking about postmenopausal
women only, not premenopausal patients. A postmenopausal woman with
ER-positive, node-negative disease,
especially if her tumor is two or three
centimeters or less, I believe has a very
good prognosis, and that’s the type of
patient in whom I would recommend
tamoxifen. I tell my patients that there’s
no question in my mind that the AIs are
more effective than tamoxifen, although
the amplification of that benefit in a
very good-prognosis patient is very
small in terms of the added proportional
reduction over tamoxifen. DR HAYES: First of all, let’s be clear
that we’re talking about postmenopausal
women only, not premenopausal patients. A postmenopausal woman with
ER-positive, node-negative disease,
especially if her tumor is two or three
centimeters or less, I believe has a very
good prognosis, and that’s the type of
patient in whom I would recommend
tamoxifen. I tell my patients that there’s
no question in my mind that the AIs are
more effective than tamoxifen, although
the amplification of that benefit in a
very good-prognosis patient is very
small in terms of the added proportional
reduction over tamoxifen.
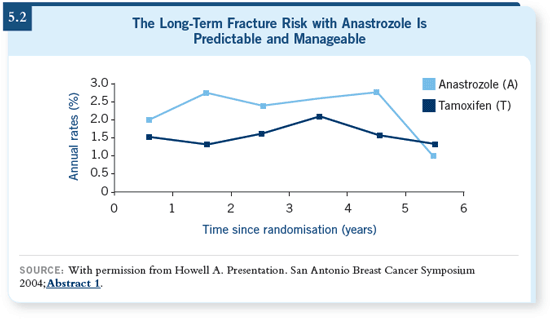
With an AI, bone fractures are a risk.
Granted, the fracture curves look like
they are coming together, but you have
to recall that there are precious few
patients out in that tail beyond five
years (5.2). That’s a very unstable statistical
tail. Most of those patients are to
the left of that, between three and four
years.
 DR LOVE: Chuck, where are you on
the debate of tamoxifen versus aromatase
inhibitors? DR LOVE: Chuck, where are you on
the debate of tamoxifen versus aromatase
inhibitors?
 DR VOGEL: I’ve listened to everybody,
and I actually agree with everything
that’s been said. We worry about the
cost issues, and there’s no question that
I treat some patients with tamoxifen
solely on the basis of cost. DR VOGEL: I’ve listened to everybody,
and I actually agree with everything
that’s been said. We worry about the
cost issues, and there’s no question that
I treat some patients with tamoxifen
solely on the basis of cost.
I think that looking at the overall
results, the aromatase inhibitors have a
better toxicity profile, except for two
areas, and they probably have slightly
more efficacy. On the other hand, they
are more costly.
So, in practice, I discuss all of these
things with my patients. I have no
problem if a patient elects to receive
tamoxifen because of cost or whatever.
It’s a very good drug. But, cost
notwithstanding — and with the
proviso that I don’t like starting the
osteoporotic patients on aromatase
inhibitors — I start with an aromatase
inhibitor. I switch from tamoxifen to an
aromatase inhibitor at two years, and I
switch to letrozole at five years.
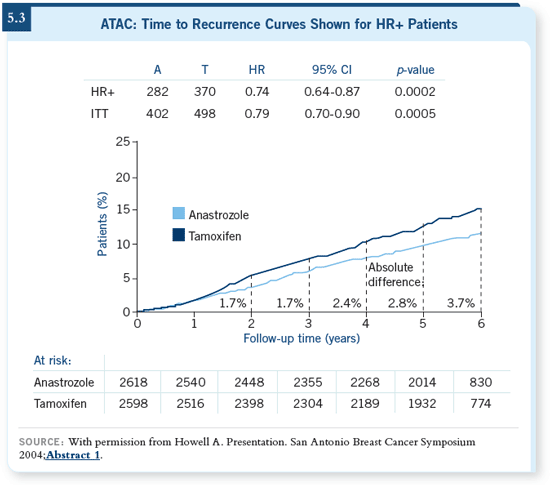
 DR RAVDIN: I absolutely agree with the
ASCO Technology Assessment statement
that basically, for postmenopausal
women with ER-positive disease, an
aromatase inhibitor should be part of
their adjuvant hormonal therapy (Winer
2005). I think it’s quite clear that these agents reduce the risk of relapse. Every
single one of the major trials, all five
of them, show marked reduction in the
risk of relapse with aromatase inhibitors. DR RAVDIN: I absolutely agree with the
ASCO Technology Assessment statement
that basically, for postmenopausal
women with ER-positive disease, an
aromatase inhibitor should be part of
their adjuvant hormonal therapy (Winer
2005). I think it’s quite clear that these agents reduce the risk of relapse. Every
single one of the major trials, all five
of them, show marked reduction in the
risk of relapse with aromatase inhibitors.
 DR CHLEBOWSKI: Looking at the
toxicity profile and the fact that you’re
getting more recurrences if you start
with tamoxifen first, I would rather
start with an aromatase inhibitor and
have fewer relapses, because those are
not recoverable in a major sense. DR CHLEBOWSKI: Looking at the
toxicity profile and the fact that you’re
getting more recurrences if you start
with tamoxifen first, I would rather
start with an aromatase inhibitor and
have fewer relapses, because those are
not recoverable in a major sense.
I think it’s possible that shorter durations
of hormone therapies will be better.
We had more signals on that at ASCO
this year with the two- versus five-year
tamoxifen randomized trial showing
absolutely no difference in survival after
10 years of follow-up (Valentini 2005).
 DR RAVDIN: The clinical data are
what drive things. And the same way
that nobody could anticipate what the
combined arm of ATAC was going
to show, we don’t know what will be
revealed with these switching strategies. DR RAVDIN: The clinical data are
what drive things. And the same way
that nobody could anticipate what the
combined arm of ATAC was going
to show, we don’t know what will be
revealed with these switching strategies.
 DR CHLEBOWSKI: I agree that the
empirical data will rule, but what
happens is you’re going to be behind for
sure in terms of recurrences if you start
with tamoxifen first, and in many cases
that’s a terminal disease that you can’t
recover from. I think you’ll have time
to see what the empirical data will be
for the majority of your patients because
we’ll get some kind of signal from
BIG FEMTA. We’ll have that result in
probably three years. DR CHLEBOWSKI: I agree that the
empirical data will rule, but what
happens is you’re going to be behind for
sure in terms of recurrences if you start
with tamoxifen first, and in many cases
that’s a terminal disease that you can’t
recover from. I think you’ll have time
to see what the empirical data will be
for the majority of your patients because
we’ll get some kind of signal from
BIG FEMTA. We’ll have that result in
probably three years.
Select publications
|

