
 |
||||||||

![]() DR LOVE: Have you treated any patients in a nonprotocol setting with
adjuvant trastuzumab?
DR LOVE: Have you treated any patients in a nonprotocol setting with
adjuvant trastuzumab?
![]() DR SLAMON: Yes, quite a few, but if we see node-negative tumors that
are less than 1.5 centimeters, it gets into a gray zone.
DR SLAMON: Yes, quite a few, but if we see node-negative tumors that
are less than 1.5 centimeters, it gets into a gray zone.
![]() DR LOVE: Wow! So you’ll treat a patient with a 1.5-cm, node-negative,
HER2-positive tumor? How long do you continue the trastuzumab?
DR LOVE: Wow! So you’ll treat a patient with a 1.5-cm, node-negative,
HER2-positive tumor? How long do you continue the trastuzumab?
![]() DR SLAMON: We use one year as in our BCIRG 006 trial, until we
obtain more data. I recently treated a patient with newly diagnosed
HER2-positive breast cancer — a young, premenopausal woman with a
1.7-cm, node-negative tumor. Her biggest concern was her heart because
she wanted to continue to be a competitive runner.
DR SLAMON: We use one year as in our BCIRG 006 trial, until we
obtain more data. I recently treated a patient with newly diagnosed
HER2-positive breast cancer — a young, premenopausal woman with a
1.7-cm, node-negative tumor. Her biggest concern was her heart because
she wanted to continue to be a competitive runner.
I explained to her that this would be off-study therapy — that this is not the way to answer the question, and we need to answer the question scientifically — but for her individual case, understanding all the caveats, we would recommend TCH. She chose to be treated and is now out three years and doing fine.
![]() DR LOVE: So you are offering adjuvant trastuzumab outside a protocol
setting, even for patients with node-negative disease?
DR LOVE: So you are offering adjuvant trastuzumab outside a protocol
setting, even for patients with node-negative disease?

![]() DR SLAMON: My belief is that a HER2-
positive tumor is a HER2-positive tumor —
nodes involved, nodes not involved, it doesn’t
matter. Clearly, the right way to answer the
question, Neil, is in a study.
DR SLAMON: My belief is that a HER2-
positive tumor is a HER2-positive tumor —
nodes involved, nodes not involved, it doesn’t
matter. Clearly, the right way to answer the
question, Neil, is in a study.
We tell the patient what we know and also that this depends on whether the payer will pay, because we don’t want to put that burden on a patient.
But for the patient who’s coming to you now with a HER2-positive tumor and can’t wait six years for an answer from the studies, we are open to off-protocol therapy.
![]() DR LOVE: What about the potential for cardiac toxicity?
DR LOVE: What about the potential for cardiac toxicity?
During this conversation five years ago, I distinctly remember my eyes opening wide with surprise as Dennis Slamon reeled off some of the boldest comments recorded during my 20-year CME journey. At the time of the interview and right up until the data explosions at the 2005 ASCO meeting, virtually every other breast cancer clinical investigator worshipped at the altar of the evidence base and steadfastly recommended against the nonprotocol use of adjuvant trastuzumab.
Our Patterns of Care studies during that time also consistently demonstrated that the vast majority of practicing oncologists were following the advice of their investigator colleagues, relegating adjuvant trastuzumab to trial use only. As evidenced in the aforementioned comments, the father of anti-HER2 therapy was one of the notable exceptions to that line of thinking.
On this issue of our series, we are reminded that as is frequently the case, Father knows best. To make that point, I asked Dr Slamon’s UCLA colleague Dr Mark Pegram and new Emory arrival and “Hotlanta” resident Dr Brian Leyland-Jones to present patients from their practices who typify how in 2007 they approach some of the most challenging issues in adjuvant therapy for HER2-positive disease.
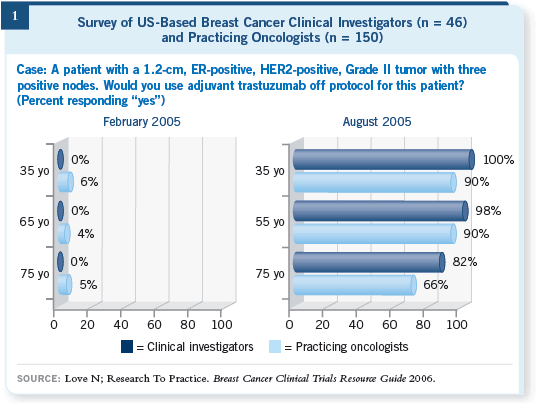
Among the cases discussed were two women with 0.5-cm, node-negative tumors who received trastuzumab. Five years ago, this situation was a “gray zone” for even Dr Slamon, and just two years ago, patients with multiple node-positive disease would be unlikely to receive anti-HER2 therapy outside of a study. But the rapidity of change in clinical practice with regard to trastuzumab is unprecedented in recent breast oncology history (Figure 1).
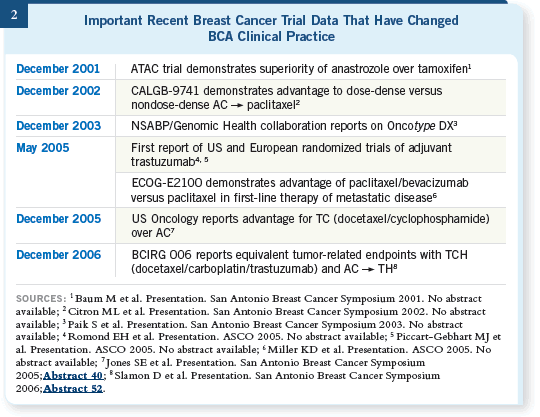
Over the years, we have observed that most important advances in the BCA clinical practice (Figure 2) have taken root much more gradually than with trastuzumab, and even a year after the initial presentation of most of these groundbreaking studies, less than a third of oncologists had changed their practices accordingly. With time, however, clinicians incorporated all of these innovations into daily patient care.
Adjuvant trastuzumab was much different, and the almost instantaneous use of this new treatment strategy was multifactorial but concentrated on two important factors: (1) the data were impressive and highly credible, and (2) everyone expected the trials to be positive based on the background research in metastatic disease. Now that we have quickly moved past the paradigm shift toward adjuvant trastuzumab, it will be fascinating to observe what people do with the latest piece of the HER2 puzzle, Dr Slamon’s provocative presentation of the second data analysis of BCIRG 006 during the December 2006 San Antonio Breast Cancer Symposium.
As is often the case with presentations by this legendary investigator, the conclusions were controversial — specifically that, in essence, TCH should be the new standard adjuvant treatment for HER2-positive disease and that the time has come to put the nail into the anthracycline/trastuzumab coffin.
A think tank held by our group one month later demonstrated an immediate rift in the clinical research community on this issue, and BCIRG standard bearer John Mackey seemed perplexed that so many at the table were still hanging on to their anthracycline-based security blankets. To this end, I can’t wait to see how investigators, community oncologists and patients react to the new BCIRG-NSABP trial that randomly assigns patients with HER2-positive disease to TCH alone or with bevacizumab.
Anyone can administer penicillin for pneumococcal pneumonia, but it takes a master physician to sort through the increasingly complex tumor biology and menu of options in contemporary oncology and be as objective as possible in making crucial life recommendations for patients. As part of that painstaking process, we sometimes choose to weigh the risks involved and educate our patients about the unknowns in order to walk outside the normal barriers of decision-making.
The wisdom and courage that this requires sometimes yield highly satisfying results, and in that regard, somewhere in the hills outside Los Angeles, a woman glides effortlessly on her daily jog, silently aware that the uncertain steps she and her physician took some years ago may have avoided the nightmare of cancer recurrence.
Neil Love, MD
NLove@ResearchToPractice.com
April 13, 2007