
 |
||||||||

| Tracks 1-12 | ||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Would you discuss the bone data from the ATAC trial
presented at the 2006 ASCO meeting?
DR LOVE: Would you discuss the bone data from the ATAC trial
presented at the 2006 ASCO meeting?
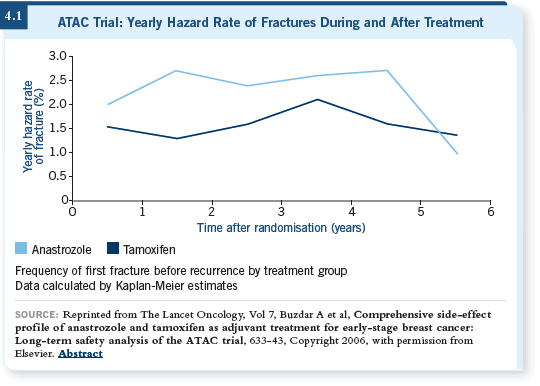
![]() DR HOWELL: The bone data (Coleman 2006; ATAC 2006; [4.1]) are important
because these are the first five-year data on the aromatase inhibitors,
and they show a marked loss on anastrozole compared to tamoxifen over the
five years in ATAC — a seven to eight percent loss over the five years in the
lumbar spine, but this levels off slightly after two years. In the hip, it goes
straight to eight percent.
DR HOWELL: The bone data (Coleman 2006; ATAC 2006; [4.1]) are important
because these are the first five-year data on the aromatase inhibitors,
and they show a marked loss on anastrozole compared to tamoxifen over the
five years in ATAC — a seven to eight percent loss over the five years in the
lumbar spine, but this levels off slightly after two years. In the hip, it goes
straight to eight percent.
It does appear that we are able to prevent bone loss with the bisphosphonates, but the important clinical point from the analysis was that if they started with a normal bone density, none of those women became osteoporotic over the five years. If the patient starts with osteopenia, you need to follow her carefully.
![]() DR LOVE: What do we know right now about how effective bisphosphonates
are in preventing this bone loss?
DR LOVE: What do we know right now about how effective bisphosphonates
are in preventing this bone loss?
![]() DR HOWELL: We’re beginning to get quite a lot of data. The most important
data remain those from the Austrian study, which was published in the Journal
of Clinical Oncology (Gnant 2007). They show that zoledronic acid at four
milligrams administered every six months completely abrogated the bone loss
from goserelin with either tamoxifen or anastrozole. This was presented at San
Antonio two years ago by Michael Gnant (Gnant 2004).
DR HOWELL: We’re beginning to get quite a lot of data. The most important
data remain those from the Austrian study, which was published in the Journal
of Clinical Oncology (Gnant 2007). They show that zoledronic acid at four
milligrams administered every six months completely abrogated the bone loss
from goserelin with either tamoxifen or anastrozole. This was presented at San
Antonio two years ago by Michael Gnant (Gnant 2004).
Track 3
![]() DR LOVE: Can you describe the initial results of the EFECT study for
postmenopausal patients with hormone receptor-positive metastatic disease
(Gradishar 2006; [2.1])?
DR LOVE: Can you describe the initial results of the EFECT study for
postmenopausal patients with hormone receptor-positive metastatic disease
(Gradishar 2006; [2.1])?
![]() DR HOWELL: The trial compared fulvestrant to exemestane after failure on a
previous nonsteroidal aromatase inhibitor as second- or third-line therapy in
advanced disease, and patients could enroll after having received an aromatase
inhibitor as adjuvant therapy.
DR HOWELL: The trial compared fulvestrant to exemestane after failure on a
previous nonsteroidal aromatase inhibitor as second- or third-line therapy in
advanced disease, and patients could enroll after having received an aromatase
inhibitor as adjuvant therapy.
The response rate and clinical benefit were identical between the two arms, with approximately a 20 percent response rate in each arm (2.1). At the moment, from that trial, no difference is evident between fulvestrant and exemestane with regard to time to progression. So if you want to use a simple treatment, you can administer exemestane. Otherwise, you can administer the fulvestrant injections.
Three other studies evaluating fulvestrant are important: SoFEA, SWOG-S0226 and FACT. The FACT and the SWOG studies are evaluating the combination of anastrozole and fulvestrant versus anastrozole alone after failure of a SERM such as tamoxifen, and these studies are crucially important.
The SoFEA trial is different. It’s a three-arm study: exemestane versus fulvestrant versus fulvestrant with anastrozole. That trial will provide another indication of whether fulvestrant is better than exemestane and whether the combination is better than a single agent. That trial is trying to examine whether maintaining a low estrogen environment is beneficial for the effectiveness of fulvestrant.
Track 4
![]() DR LOVE: Can you discuss the mechanism of action of fulvestrant and
the aromatase inhibitors, what you think would happen with the two in
combination and how that might be different combining a SERM like
tamoxifen with an aromatase inhibitor?
DR LOVE: Can you discuss the mechanism of action of fulvestrant and
the aromatase inhibitors, what you think would happen with the two in
combination and how that might be different combining a SERM like
tamoxifen with an aromatase inhibitor?
![]() DR HOWELL: The major difference between tamoxifen and fulvestrant is
thought to be what happens when the SERM or the SERD binds the estrogen
receptor. When tamoxifen binds the receptor, it goes to the estrogen response
element (ERE) of the appropriate genes.
DR HOWELL: The major difference between tamoxifen and fulvestrant is
thought to be what happens when the SERM or the SERD binds the estrogen
receptor. When tamoxifen binds the receptor, it goes to the estrogen response
element (ERE) of the appropriate genes.
Tamoxifen can inhibit the gene activity, but the estrogen receptor can also be activated by growth factors through phosphorylation of activating factor one (AF-1). The SERD causes downregulation of the receptor, which is degraded, and growth factors can’t act through phosphorylating AF-1.
With the combination, the aromatase inhibitor keeps estrogen low and inhibits it from going to the ERE. If estrogen reaches the ERE, fulvestrant causes degradation of the estrogen receptor, so presumably growth factor activity can’t occur via AF-1 and phosphorylation. The combination works in Angela Brody’s animal models, but it will be another year before we see the results of these trials and find out whether it works in women with breast cancer.
![]() DR LOVE: I guess the key issue is that fulvestrant is a competitive inhibitor of
estradiol. So you can increase the dose of fulvestrant or remove the ligand.
DR LOVE: I guess the key issue is that fulvestrant is a competitive inhibitor of
estradiol. So you can increase the dose of fulvestrant or remove the ligand.
![]() DR HOWELL: Exactly. That is the rationale behind the SoFEA trial. You’re
maintaining a low estrogen level in that trial, so you reduce the estrogen level
or you increase the dose or, possibly, both.
DR HOWELL: Exactly. That is the rationale behind the SoFEA trial. You’re
maintaining a low estrogen level in that trial, so you reduce the estrogen level
or you increase the dose or, possibly, both.
Track 7
![]() DR LOVE:Can you discuss where we are with the IBIS-1 and Royal
Marsden studies?
DR LOVE:Can you discuss where we are with the IBIS-1 and Royal
Marsden studies?
![]() DR HOWELL: The data on long-term preventive effects of tamoxifen are clearly
important. Four prevention trials are under way, but only two of them will be
able to provide good long-term data that are uncontaminated by women who were on the control arm receiving treatment, as occurred in NSABP-P-1.
DR HOWELL: The data on long-term preventive effects of tamoxifen are clearly
important. Four prevention trials are under way, but only two of them will be
able to provide good long-term data that are uncontaminated by women who were on the control arm receiving treatment, as occurred in NSABP-P-1.
Clearly NSABP-P-1 is an important study with large numbers, and within a relatively short period of treatment, patients had a 50 percent reduction in the risk of developing breast cancer.
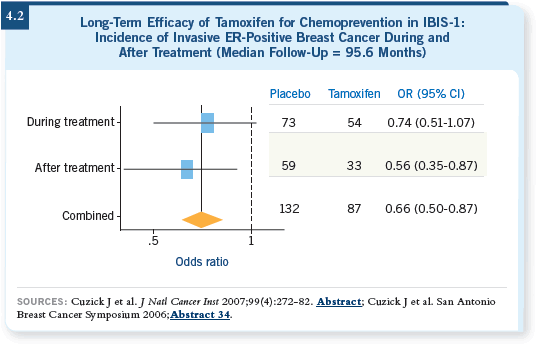
Two studies (Cuzick 2006; Powles 2006) reported at San Antonio asked the question, what happens when you stop treatment? Would you see a carryover effect or not? The answer is that you do see a carryover effect, which is absolutely fascinating. In the IBIS-1 study, 7,000 women remained blinded after stopping five years of treatment, and the data presented by Jack Cuzick demonstrated a 30 to 40 percent reduction continued after 10 years (4.2) — and the toxicity goes down (Cuzick 2006).
The problem of endometrial cancer goes away, and the deep vein thrombosis goes away (Sestak 2006). So you’re left with a net benefit in the end. Therefore, let’s reconsider the view that we have on tamoxifen and reconsider this drug as a preventative agent.
The data are supported by the Royal Marsden trial, which Trevor Powles presented (Powles 2006). It was a randomized trial of eight years of tamoxifen versus placebo. He’s shown a 50 percent reduction beyond stopping tamoxifen, which is clearly another carryover effect. So we have two trials showing a carryover effect, which is important for prevention.
Track 8
![]() DR LOVE: Can you discuss the IBIS-2 and MAP-3 trials?
DR LOVE: Can you discuss the IBIS-2 and MAP-3 trials?
![]() DR HOWELL: IBIS-2 and MAP-3 are logical trials to conduct. IBIS-2 evaluates
anastrozole versus placebo, and MAP-3 compares exemestane to placebo
in postmenopausal women at increased risk of developing breast cancer. Both
trials are recruiting reasonably well. IBIS-2 needs 6,000 women, and MAP-3
needs about 4,500 to 5,000 women.
DR HOWELL: IBIS-2 and MAP-3 are logical trials to conduct. IBIS-2 evaluates
anastrozole versus placebo, and MAP-3 compares exemestane to placebo
in postmenopausal women at increased risk of developing breast cancer. Both
trials are recruiting reasonably well. IBIS-2 needs 6,000 women, and MAP-3
needs about 4,500 to 5,000 women.
The important data presented at the San Antonio meeting from the point of view of IBIS-2 are on cognitive function. I was particularly worried that a decline in cognitive function would appear with anastrozole, and we’ve shown in more than 200 patients that after six months there’s absolutely no difference in cognitive function, and these women underwent 10 carefully controlled cognitive function tests ( Jenkins 2006). I find this reassuring. The patients will receive up to five years of follow-up. They’ll undergo another test at two years and another at five years.