
 |
||||||||

On this edition of Breast Cancer Update, Dr Joseph Sparano chats about two of the most important breast cancer clinical trials of the last decade, both of which are fortunate to have him as their principal investigator.
The first is ECOG-E1199, which was launched in 1999, at a time when the optimal use of taxanes as adjuvant therapy was an issue of great concern. Under Dr Sparano’s able leadership, this simply designed trial quickly accrued more than 5,000 patients, who received AC followed by either docetaxel or paclitaxel every week or three weeks.
As discussed in the interview, like many other adjuvant trials in recent years, E1199 ended up having fewer recurrences and deaths than anticipated. After many months of waiting, the Data Safety and Monitoring Committee decided to recommend release of the results before the stipulated number of events had occurred.
ECOG-E1199 was first reported at the 2005 San Antonio Breast Cancer Symposium by Dr Sparano, causing a considerable stir. To many observers’ surprise, this two-by-two design didn’t demonstrate a great deal of difference between the regimens, but weekly paclitaxel seemed to have the best risk-benefit ratio, although every three-week docetaxel appeared comparable.
The problem is that, thankfully, in the seven years since the trial launched, a lot
has happened in research on adjuvant therapy of breast cancer, particularly the
release of results of CALGB-9741 and BCIRG 001, demonstrating advantages
for dose-dense AC ![]() paclitaxel and TAC, respectively. Another critical development
in research on adjuvant chemotherapy was the US Oncology trial led
by Steve Jones, which demonstrated superiority in efficacy and tolerability
of TC (docetaxel/cyclophosphamide) compared to AC. These regimens are
now frequently utilized by medical oncologists and have made the E1199 data
somewhat less exciting than was hoped for when the trial was designed.
paclitaxel and TAC, respectively. Another critical development
in research on adjuvant chemotherapy was the US Oncology trial led
by Steve Jones, which demonstrated superiority in efficacy and tolerability
of TC (docetaxel/cyclophosphamide) compared to AC. These regimens are
now frequently utilized by medical oncologists and have made the E1199 data
somewhat less exciting than was hoped for when the trial was designed.
The recent availability of nanoparticle albumin-bound (nab) paclitaxel is another development that is interesting in light of E1199. I am starting to hear a consistent response when breast cancer investigators opine about nab. The bottom line is that many would throw old-fashioned paclitaxel (Pac) and its Cremophor® base right in the garbage if cost were not an issue. Of interest is our recent Patterns of Care survey, in which 83 percent of breast cancer investigators and 73 percent of practicing oncologists believe that nab has a greater antitumor effect than its close cousin, Pac, yet the same survey shows that not that much nab is being utilized — mainly because of cost. I wonder what patients would think about this.
Dr Sparano is also the principal investigator of another critical study that has been discussed and eagerly anticipated for several years. TAILORx (aka the PACCT-1 Trial) features a design that only five years ago would have seemed like science fiction. Following the landmark collaboration between Soon Paik of the NSABP and Steve Shak of Genomic Health, patients entering TAILORx will be randomly assigned to one of three study arms based on their Oncotype DX™ recurrence score (Figure 1).

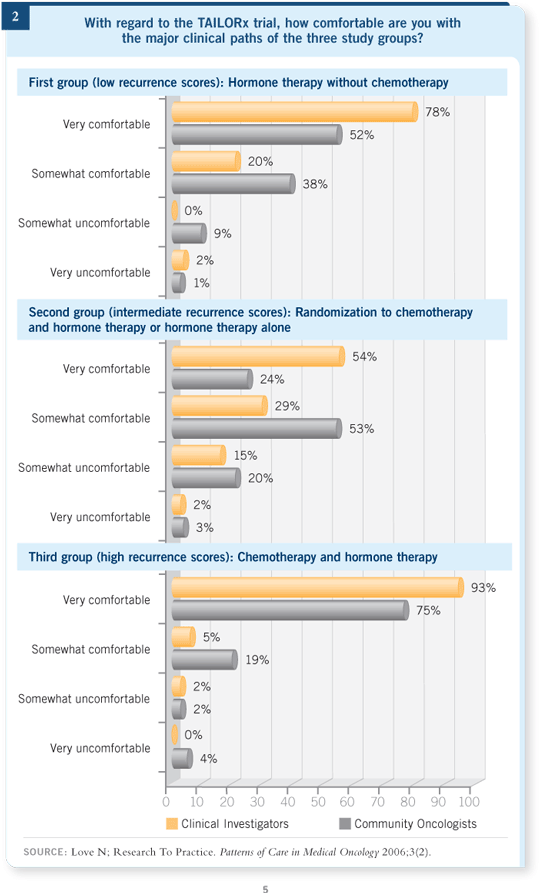
For the first time in a long, long time, this adjuvant study uses a chemotherapy versus no chemotherapy randomization. Although this may seem controversial, our Patterns of Care study suggests that oncologists are mostly comfortable with the idea of entering patients on this landmark study (Figure 2).
We can only speculate about how TAILORx will be viewed five years from now, but for what it’s worth, my bet is that even if the clinical questions being addressed become outdated (as sort of happened with E1199), the careful molecular study of tumors is here to stay, and the correlation with superbly documented follow-up as is being done in this study will provide biologic and therapeutic insights about breast cancer that will shape the next generation of interventions.
I recently spent 90 amazing minutes recording an interview with Soon Paik, and when it was over, my brain hurt. When I told Soon that he reminded me of a physics professor teaching a course I was destined to fail, he chuckled humbly and told me that he didn’t understand genomics and proteomics either. Yeah, right.
Cancer patients and their loved ones are relying on geniuses like Soon and Steve Shak to jump-start oncologic research and on clinical leaders like Joe Sparano to take state-of-the-art technologies like Oncotype and rally our team to get trials done quickly, and maybe turn this nasty disease into a bad memory.
— Neil Love, MD
NLove@ResearchToPractice.com
January 15, 2007
SELECT PUBLICATIONS
Citron ML et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 2003;21(8):1431-9. Abstract
Gradishar W et al. A randomized phase 2 trial of qw or q3w ABI-007 (ABX) vs q3w solvent-based docetaxel (TXT) as first-line therapy in metastatic breast cancer (MBC). San Antonio Breast Cancer Symposium 2006;Abstract 46.
Gradishar WJ et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 2005;23(31):7794-803. Abstract
Hudis C et al. Five year follow-up of INT C9741: Dose-dense (DD) chemotherapy (CRx) is safe and effective. San Antonio Breast Cancer Symposium 2005;Abstract 41.
Martin M et al; Breast Cancer International Research Group 001 Investigators. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 2005;352(22):2302-13. Abstract
Paik S et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006;24(23):3726-34. Abstract
Paik S et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351(27):2817-26. Abstract
Sparano JA et al. Phase III study of doxorubicin-cyclophosphamide followed by paclitaxel or docetaxel given every 3 weeks or weekly in patients with axillary node-positive or high-risk node-negative breast cancer: Results of North American Breast Cancer Intergroup Trial E1199. San Antonio Breast Cancer Symposium 2005;Abstract 48.