
 |
||||||||

| Tracks 1-17 | ||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 2
![]() DR LOVE: Can you discuss data from your toxicity analysis of chemotherapy
in older patients who participated in CALGB studies?
DR LOVE: Can you discuss data from your toxicity analysis of chemotherapy
in older patients who participated in CALGB studies?
![]() DR MUSS: We analyzed data from three Cancer and Leukemia Group B
clinical trials that were conducted over a span of years (Muss 2006), including
CALGB-9344, which compared AC with or without paclitaxel. We previously
had shown that older women in these studies derived the same proportional benefit as the younger patients in terms of relapse-free survival and overall
survival (Muss 2005).
DR MUSS: We analyzed data from three Cancer and Leukemia Group B
clinical trials that were conducted over a span of years (Muss 2006), including
CALGB-9344, which compared AC with or without paclitaxel. We previously
had shown that older women in these studies derived the same proportional benefit as the younger patients in terms of relapse-free survival and overall
survival (Muss 2005).
For instance, a woman older than 65 years of age who was receiving the paclitaxel-containing regimen after AC experienced similar benefits from paclitaxel to those the younger patients experienced in this study, in aggregate. Therefore, we feel that age was not a variable predictive of benefit from chemotherapy. These trials all comprised highly selected patients with node-positive disease.
We went back and assessed detailed toxicity in three of the clinical trials. Of approximately 6,500 patients, only seven percent were 65 years and older and only three percent were 70 and older. It sounds as if those numbers were small, but when you consider what’s out there, these are some of the largest numbers of patients analyzed prospectively in a trial.
![]() DR LOVE: How many treatment-related deaths did you find?
DR LOVE: How many treatment-related deaths did you find?
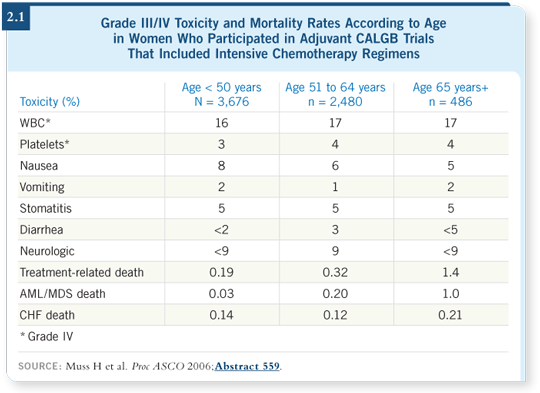
![]() DR MUSS: Approximately 24 deaths were attributed to therapy (2.1). When
you run a large clinical trial and a patient gets neutropenic fever and dies in
the hospital, it’s a catastrophe. It’s obviously therapy related. But if someone
dies of heart trouble four years after receiving an anthracycline-containing
therapy, is that related? The 24 deaths attributed to therapy were coded by the
principal investigator. It was his or her decision whether or not a death was
treatment related.
DR MUSS: Approximately 24 deaths were attributed to therapy (2.1). When
you run a large clinical trial and a patient gets neutropenic fever and dies in
the hospital, it’s a catastrophe. It’s obviously therapy related. But if someone
dies of heart trouble four years after receiving an anthracycline-containing
therapy, is that related? The 24 deaths attributed to therapy were coded by the
principal investigator. It was his or her decision whether or not a death was
treatment related.
Comparing those numbers with the size of the groups being treated, we found treatment-related death occurred in 1.5 percent of women age 65 and older, but the confidence interval was wide.
If you calculate trend statistics, the older patients have a higher probability of dying as a result of treatment. We also wanted to see if we could figure out a way to determine whether older people could be dying later on of treatment-related toxicity, such as cardiac disease, leukemia, et cetera.
For leukemia, it’s interesting. Of the 24 deaths, we didn’t have one septic death attributed to treatment in these three trials. That was probably good luck because in larger trials, one or two patients always die as a result of a defined, well-used adjuvant program, but we didn’t see it here.
We saw a substantial number of patients with acute leukemia, and they tended to be older patients. The leukemia tended to fit in the right time range — five to 10 years, which is when you expect treatment-related leukemia to occur. Factoring this out, the elderly had a higher risk of leukemia than the younger patients. Of the seven older patients who died of treatment-related causes, five deaths were from leukemia.
![]() DR LOVE: If a 70-year-old woman who is receiving dose-dense AC and paclitaxel
asks you her chances of developing treatment-related leukemia, what do
you say?
DR LOVE: If a 70-year-old woman who is receiving dose-dense AC and paclitaxel
asks you her chances of developing treatment-related leukemia, what do
you say?
![]() DR MUSS: It’s about a half to one percent. In Dr Hudis’s update (Hudis 2005) of CALGB-9741, which compared the dose-dense regimen with the every
three-week regimen, the incidence of AML/MDS was 0.7 percent, which is
substantial.
DR MUSS: It’s about a half to one percent. In Dr Hudis’s update (Hudis 2005) of CALGB-9741, which compared the dose-dense regimen with the every
three-week regimen, the incidence of AML/MDS was 0.7 percent, which is
substantial.
Track 4
![]() DR LOVE: Would you discuss CALGB-49907?
DR LOVE: Would you discuss CALGB-49907?
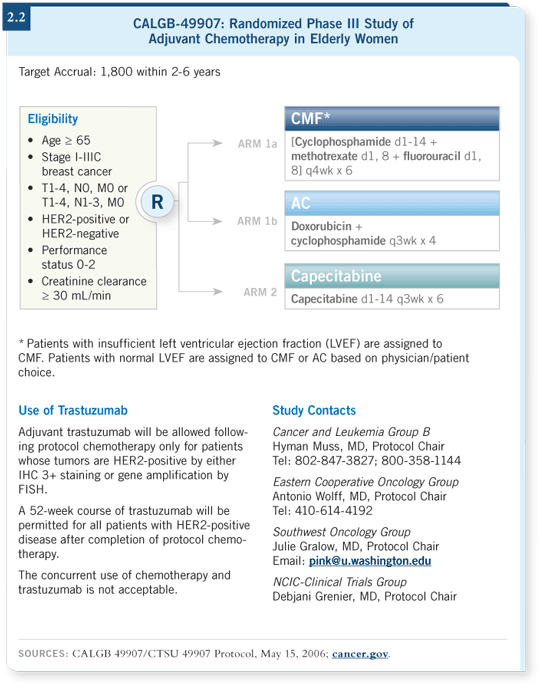
![]() DR MUSS: We’re conducting a clinical trial through the Intergroup, CTSU
with CALGB, evaluating chemotherapy with the oral agent capecitabine
versus standard therapy for women age 65 years and older. Standard therapy
in this trial is either CMF using an oral cyclophosphamide regimen for six
months or AC for four cycles. Capecitabine is administered at a dose of 2,000
mg/m2 for 14 days out of every 21 days for six cycles (2.2).
DR MUSS: We’re conducting a clinical trial through the Intergroup, CTSU
with CALGB, evaluating chemotherapy with the oral agent capecitabine
versus standard therapy for women age 65 years and older. Standard therapy
in this trial is either CMF using an oral cyclophosphamide regimen for six
months or AC for four cycles. Capecitabine is administered at a dose of 2,000
mg/m2 for 14 days out of every 21 days for six cycles (2.2).
We’ve enrolled 600 patients, which is our first cutoff for a Bayesian analysis to assess whether to continue the trial or stop it. The Data Safety Monitoring Board will evaluate the data and the event rates.
If it seems highly improbable that the capecitabine regimen will be less effective than standard therapy, the study will be stopped. If it’s a “slam dunk” that either AC or CMF is better than capecitabine, then the study will be stopped. If the results are in the middle, we will go on to accrue several hundred more patients. We hope this analysis will be completed over the next several months and that the data will be helpful.
Track 10
![]() DR LOVE: What are your thoughts about the work that’s coming out of
Memorial Sloan-Kettering, evaluating capecitabine one week on and one
week off — the so-called dose-dense capecitabine?
DR LOVE: What are your thoughts about the work that’s coming out of
Memorial Sloan-Kettering, evaluating capecitabine one week on and one
week off — the so-called dose-dense capecitabine?
![]() DR MUSS: I’ve seen some of the preclinical data, and they’re intriguing.
Maybe the dose-dense approach is a better way to administer capecitabine. I
believe capecitabine is extremely effective.
DR MUSS: I’ve seen some of the preclinical data, and they’re intriguing.
Maybe the dose-dense approach is a better way to administer capecitabine. I
believe capecitabine is extremely effective.
We’ve used a fixed dose of capecitabine. I believe there’s a lot more we can learn about this drug. For instance, would capecitabine be a good choice for metronomic low-dose chemotherapy to administer over a long period? I’m not sure we know the optimal dose or threshold dose of capecitabine.
I treated one woman with metastatic disease who was in her eighties with 500 milligrams BID because she said, “If I get the least bit sick from your treatment, I’m never coming back to see you.” She had extensive pulmonary metastases, so I figured I could risk it.
She had a response that lasted about nine months, and when she came back without toxicity, I said maybe if we pushed up the dose, we’d do better. Of course, she was logical and said, “Why? Why would you want to do it?”
We still have a lot of fundamental biology to learn about capecitabine, and there may be better ways to administer it. Perhaps the dose-dense approach, which follows much of Larry Norton’s work and mathematic models, will be a better way.
![]() DR LOVE: What about the research question of adding capecitabine to some of
the existing regimens — for example, following dose-dense AC
DR LOVE: What about the research question of adding capecitabine to some of
the existing regimens — for example, following dose-dense AC![]() paclitaxel?
paclitaxel?
![]() DR MUSS: Trials are in progress that utilize capecitabine in addition to
taxanes. There is a US Oncology trial of AC followed by docetaxel or
capecitabine/docetaxel, which leans on the Phase III data that have been
published by Joyce O’Shaughnessy (O’Shaughnessy 2002).
DR MUSS: Trials are in progress that utilize capecitabine in addition to
taxanes. There is a US Oncology trial of AC followed by docetaxel or
capecitabine/docetaxel, which leans on the Phase III data that have been
published by Joyce O’Shaughnessy (O’Shaughnessy 2002).
When you’re talking about response and potentially curable patients in the adjuvant setting, the combination makes a lot of sense.
So that’s a great setting in which to explore capecitabine up front, in addition to other agents, and perhaps to consider nonanthracycline/capecitabine regimens with taxanes or gemcitabine or with other agents that we traditionally haven’t used but that could be highly effective.
Track 11
![]() DR LOVE: Prior to the presentation of the paclitaxel/bevacizumab data at
ASCO 2005 (Miller 2005a), we were seeing capecitabine used by clinical
investigators a lot more in metastatic disease as first-line therapy compared
to practicing oncologists. Now questions have arisen because of the paclitaxel/
bevacizumab data. How have you sorted through that?
DR LOVE: Prior to the presentation of the paclitaxel/bevacizumab data at
ASCO 2005 (Miller 2005a), we were seeing capecitabine used by clinical
investigators a lot more in metastatic disease as first-line therapy compared
to practicing oncologists. Now questions have arisen because of the paclitaxel/
bevacizumab data. How have you sorted through that?
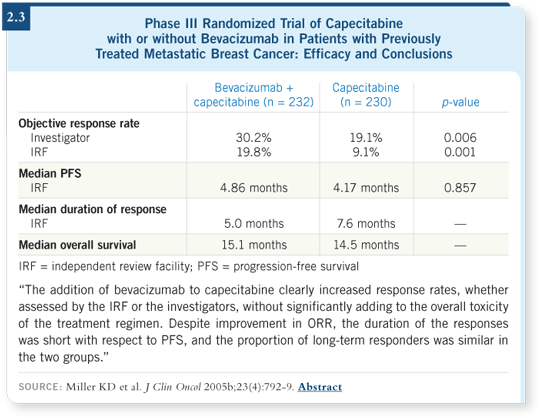
![]() DR MUSS: That’s a great question. Kathy Miller reported a trial comparing
capecitabine/bevacizumab to capecitabine alone (Miller 2005b), but for virtually
all those patients, it was second-line or later therapy.
DR MUSS: That’s a great question. Kathy Miller reported a trial comparing
capecitabine/bevacizumab to capecitabine alone (Miller 2005b), but for virtually
all those patients, it was second-line or later therapy.
The response rate was a little bit better with bevacizumab (2.3), but we didn’t see impressive changes in time to progression or survival in the long run, which we saw in the paclitaxel/bevacizumab trial.
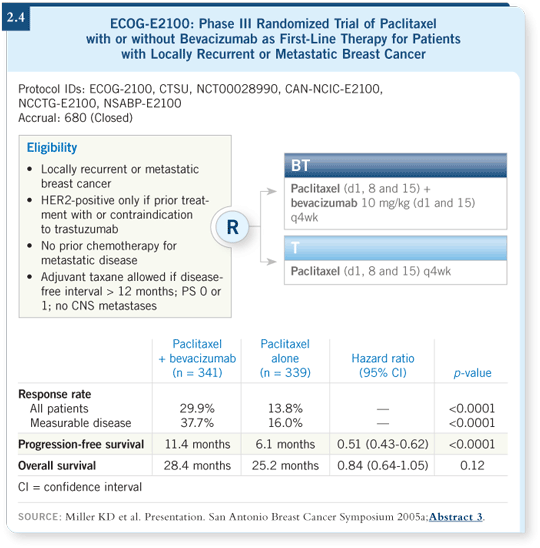
The paclitaxel/bevacizumab data are impressive and demonstrate improvements in response rate (2.4), doubling the time to progression to about 11 months — and in randomized trials with large numbers, that’s among the best time to progression data you will see.
Toxicity, hypertension and other side effects are of concern with bevacizumab, but these are manageable compared to what you see with a lot of the other chemotherapy agents.
Those data have made our decisions more difficult. Before the paclitaxel/bevacizumab trial (Miller 2005a), I would have used capecitabine as first-line treatment for most patients. The truth is, whether you’re 25 years old with metastatic breast cancer or 85 years old, it’s palliative therapy.
For someone who’s been through adjuvant therapy, who has incurable disease and who is getting used to the fact that she has a serious problem, using a drug that doesn’t cause hair loss, doesn’t usually cause myelosuppression and allows her to maintain a pretty good quality of life — when she’s just been hit with the terrible news that she has an incurable metastatic breast cancer — seems like a good way to take care of a patient.
The bevacizumab data are intriguing, but I still believe, for many patients, capecitabine has a potential role up front.
Track 13
![]() DR LOVE: At the 2006 San Antonio Breast Cancer Symposium, we tried
something new called “Design A Trial,” in which
we asked people to put forth ideas about trials they’d like to see in breast
cancer — setting aside the issue of funding. What trials would you like to
see if you had the funding?
DR LOVE: At the 2006 San Antonio Breast Cancer Symposium, we tried
something new called “Design A Trial,” in which
we asked people to put forth ideas about trials they’d like to see in breast
cancer — setting aside the issue of funding. What trials would you like to
see if you had the funding?
![]() DR MUSS: Well, I’m biased toward the elderly. I’d like to see some nonanthracycline
regimens evaluated. If capecitabine turns out to be as good as
standard therapy, or perhaps even better, I’d like to add it to a taxane and
evaluate that up front, maybe against capecitabine alone. I also believe that
studying some of the new taxane preparations, such as nab paclitaxel, which
minimizes toxicity, would be exciting.
DR MUSS: Well, I’m biased toward the elderly. I’d like to see some nonanthracycline
regimens evaluated. If capecitabine turns out to be as good as
standard therapy, or perhaps even better, I’d like to add it to a taxane and
evaluate that up front, maybe against capecitabine alone. I also believe that
studying some of the new taxane preparations, such as nab paclitaxel, which
minimizes toxicity, would be exciting.
![]() DR LOVE: What are your thoughts about nab paclitaxel as opposed to paclitaxel
in the elderly?
DR LOVE: What are your thoughts about nab paclitaxel as opposed to paclitaxel
in the elderly?
![]() DR MUSS: Nab paclitaxel is intriguing because it decreases the time of treatment
and complexity of nursing care and premedication, which is a big deal in
many offices. Biologically, it uses a better delivery system.
DR MUSS: Nab paclitaxel is intriguing because it decreases the time of treatment
and complexity of nursing care and premedication, which is a big deal in
many offices. Biologically, it uses a better delivery system.
Minimizing the risk of hypersensitivity reactions is important. All of us have seen that occasional serious reaction, even after weeks and months of taxane therapy or carboplatin. They’re why we have crash carts in clinics. I don’t want to overstate the case, but it’s important.