
 |
||||||||

| Tracks 1-26 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Meeting
Track 2
![]() DR LOVE: Norm, can you discuss NSABP-B-40?
DR LOVE: Norm, can you discuss NSABP-B-40?
![]() DR WOLMARK: In two previous preoperative chemotherapy trials, NSABPB-18 (Wolmark 2001) and NSABP-B-27 (Bear 2006), we demonstrated that
those individuals who have a pathologic complete response have, by far, the
best outcome.
DR WOLMARK: In two previous preoperative chemotherapy trials, NSABPB-18 (Wolmark 2001) and NSABP-B-27 (Bear 2006), we demonstrated that
those individuals who have a pathologic complete response have, by far, the
best outcome.
In NSABP-B-27, the addition of docetaxel to AC doubled the pathologic complete response rate, but it did not do much for distant disease or survival. It did, however, lower the rate of local recurrences. Nonetheless, those individuals who had a pathologic complete response had the best outcome relative to disease-free and overall survival (Bear 2006).
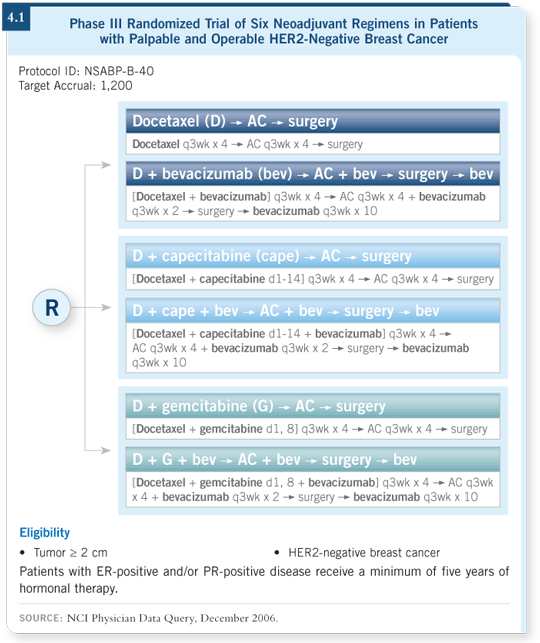
For NSABP-B-40, we decided to power the trial based on pathologic complete response and to use it to develop a molecular taxonomy. We also switched the sequence of the drugs, and we started with the taxane. The three chemotherapy arms are docetaxel followed by AC, a doublet of docetaxel/capecitabine followed by AC and a doublet of docetaxel/gemcitabine followed by AC. These chemotherapy regimens are administered with or without bevacizumab. It’s a three-by-two factorial trial design (4.1).
The novelty of NSABP-B-40 is that we’re using pCR as an endpoint with an emphasis on developing a molecular taxonomy to determine whether we can characterize patients who obtain a pCR as a surrogate marker to measure outcome. Disease-free and overall survival are not primary endpoints for NSABP-B-40.
We view it as a new mechanism to test promising agents in the neoadjuvant setting, and we believe it is an appropriate direction to pursue, particularly with the number of agents that are available and the limited resources, both from a support standpoint and a population standpoint.
Track 7
![]() DR LOVE: Steve, can you review your trial comparing docetaxel/cyclophosphamide (TC) to AC?
DR LOVE: Steve, can you review your trial comparing docetaxel/cyclophosphamide (TC) to AC?
![]() DR JONES: The objective of the trial was to compare the disease-free survival
between AC and TC for women with operable breast cancer. About half of
the women had node-negative disease and half had node-positive disease. We
recruited about 1,000 patients and had 5.5 years of median follow-up ( Jones
2006; [4.2]).
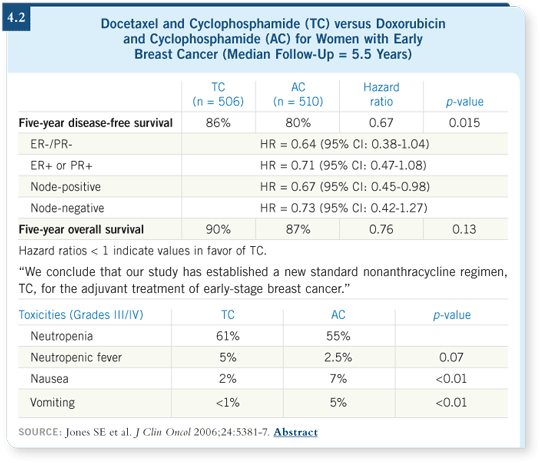
DR JONES: The objective of the trial was to compare the disease-free survival
between AC and TC for women with operable breast cancer. About half of
the women had node-negative disease and half had node-positive disease. We
recruited about 1,000 patients and had 5.5 years of median follow-up ( Jones
2006; [4.2]).
We conducted a preliminary analysis at about three years, in which a difference in favor of TC was emerging ( Jones 2003). At five years, however, this had become a significant difference, with a p-value of 0.015. We saw a one third reduction in the risk of a breast cancer event among the patients who received TC, which is a significant impact and translates into a six percent absolute difference at five years ( Jones 2006; [4.2]).
We conducted an exploratory analysis because of the interest in the differences in response to adjuvant chemotherapy between patients with hormone receptor-positive and receptor-negative disease. About 75 percent of the women had hormone receptor-positive disease. No obvious difference appeared between receptor-positive and receptor-negative disease with respect to benefit from TC ( Jones 2006; [4.2]).
A trend toward an overall survival benefit (p = 0.131) and nearly a 25 percent lower chance of dying were evident among the patients treated with TC ( Jones 2006; [4.2]). If you present it that way to patients, most will opt for TC. I believe if this trial were larger or we had longer follow-up, we might see a survival difference. The conclusion from the trial was that TC is a new standard nonanthracycline adjuvant regimen.
Personally, I would use TC in the population of patients we studied in this trial: Those with node-negative disease or those with one to three positive nodes. It provides a good reduction in the risk of recurrence.
We don’t have many data for women with four or more positive nodes, so I probably wouldn’t pick TC in those situations, but I would for the patients with lower-risk disease or those with cardiac compromise.
![]() DR BURSTEIN: For the most part, for patients with high-risk disease, I’m
using both anthracyclines and taxanes. The most common regimen we use is
dose-dense AC followed by paclitaxel. For patients with lower-risk disease,
the arguments are, do they need chemotherapy at all and what is the role of
chemotherapy?
DR BURSTEIN: For the most part, for patients with high-risk disease, I’m
using both anthracyclines and taxanes. The most common regimen we use is
dose-dense AC followed by paclitaxel. For patients with lower-risk disease,
the arguments are, do they need chemotherapy at all and what is the role of
chemotherapy?
While I find Steve’s trial very provocative and well done, I continue to use, principally, AC. We have such an enormous wealth of experience with AC, and it’s the foundation for most of the modern treatment regimens. I find it to be less toxic than TC in the short term. In addition, the side effects that most worry patients are alopecia and hospitalization risk. I think those are pretty comparable between AC and TC.
We don’t use the AC schedule used in Steve’s trial. We typically use a dose-dense schedule every two weeks. Whether that is better, I don’t know. TC is a nice option for patients for whom you want to use chemotherapy and there’s a true contraindication to an anthracycline — someone who previously received CHOP for lymphoma or had a preexisting cardiomyopathy. But it is not a regimen I use in daily practice.
![]() DR LOVE: What’s behind your decision to use dose-dense AC without a
taxane as opposed to nondose-dense AC?
DR LOVE: What’s behind your decision to use dose-dense AC without a
taxane as opposed to nondose-dense AC?
![]() DR BURSTEIN: It’s based on the assumption that it has the same efficacy and is
easier for the patients. It’s a shorter course of therapy — eight versus 12 weeks.
One of the things we learned while doing the adjuvant trastuzumab trials,
in which patients received every three-week AC, is how challenging that
regimen is to administer. In probably five to 15 percent of patients, the counts
hadn’t recovered by week three.
DR BURSTEIN: It’s based on the assumption that it has the same efficacy and is
easier for the patients. It’s a shorter course of therapy — eight versus 12 weeks.
One of the things we learned while doing the adjuvant trastuzumab trials,
in which patients received every three-week AC, is how challenging that
regimen is to administer. In probably five to 15 percent of patients, the counts
hadn’t recovered by week three.
Tracks 8-9
![]() DR LOVE: Steve, your data suggest that TC is less toxic.
DR LOVE: Steve, your data suggest that TC is less toxic.
![]() DR JONES: Our experience — and I treated a lot of patients on this trial
— was that TC was better tolerated. Many patients don’t have much nausea or
vomiting with TC (4.2), which is a big factor. A little more neutropenia does
occur.
DR JONES: Our experience — and I treated a lot of patients on this trial
— was that TC was better tolerated. Many patients don’t have much nausea or
vomiting with TC (4.2), which is a big factor. A little more neutropenia does
occur.
![]() DR RAVDIN: I believe TC should be a more popular regimen. Although we
see a lot of enthusiasm for dose-dense AC, this has never been compared to
q3wk treatment. We don’t know from the dose-dense trials which part of the
therapy is being improved. We do know that changing the schedule of the
taxanes does improve a therapy. So it may be that the additional benefit for
dose-dense therapy in CALGB-9741 came from the dose densification of the
taxane, not the anthracycline.
DR RAVDIN: I believe TC should be a more popular regimen. Although we
see a lot of enthusiasm for dose-dense AC, this has never been compared to
q3wk treatment. We don’t know from the dose-dense trials which part of the
therapy is being improved. We do know that changing the schedule of the
taxanes does improve a therapy. So it may be that the additional benefit for
dose-dense therapy in CALGB-9741 came from the dose densification of the
taxane, not the anthracycline.
Tracks 10-11
![]() DR LOVE: Hal, can you discuss your trial of dose-dense nab paclitaxel?
DR LOVE: Hal, can you discuss your trial of dose-dense nab paclitaxel?
![]() DR BURSTEIN: We recently finished accruing more than 60 patients to a
trial of AC followed by dose-dense nab paclitaxel. We had an early stopping
rule evaluating whether you could use nab paclitaxel without growth factor
support, and you cannot use nab paclitaxel every two weeks consistently
without growth factor support. Our protocol was amended so that all the
patients receive growth factor support. We’ll show these data at ASCO 2007.
DR BURSTEIN: We recently finished accruing more than 60 patients to a
trial of AC followed by dose-dense nab paclitaxel. We had an early stopping
rule evaluating whether you could use nab paclitaxel without growth factor
support, and you cannot use nab paclitaxel every two weeks consistently
without growth factor support. Our protocol was amended so that all the
patients receive growth factor support. We’ll show these data at ASCO 2007.
![]() DR LOVE: Bill, what are your thoughts about nab in breast cancer?
DR LOVE: Bill, what are your thoughts about nab in breast cancer?
![]() DR HARWIN: If nab paclitaxel were proven to have less neuropathy down
the road, that would be much more important than the premedications.
Many patients who receive paclitaxel for breast cancer and other diseases are
bothered by numbness in their extremities many months and even years later.
If nab paclitaxel were found in a large trial not to cause that, then that might
be a worthwhile advantage.
DR HARWIN: If nab paclitaxel were proven to have less neuropathy down
the road, that would be much more important than the premedications.
Many patients who receive paclitaxel for breast cancer and other diseases are
bothered by numbness in their extremities many months and even years later.
If nab paclitaxel were found in a large trial not to cause that, then that might
be a worthwhile advantage.
![]() DR LOVE: Steve, it’s been said that the neuropathy associated with nab paclitaxel
resolves more quickly.
DR LOVE: Steve, it’s been said that the neuropathy associated with nab paclitaxel
resolves more quickly.
![]() DR JONES: That does appear to be the case. It has been reported to be
relatively rapidly reversible compared to the neuropathy associated with
paclitaxel or docetaxel, which doesn’t go away (Gradishar 2005). However, I
believe the jury is still out.
DR JONES: That does appear to be the case. It has been reported to be
relatively rapidly reversible compared to the neuropathy associated with
paclitaxel or docetaxel, which doesn’t go away (Gradishar 2005). However, I
believe the jury is still out.
Track 16
![]() DR LOVE: Peter, would the Oncotype DX assay fit into this patient’s situation?
DR LOVE: Peter, would the Oncotype DX assay fit into this patient’s situation?
![]() DR RAVDIN: I believe the Oncotype DX assay could be used for a patient like
this. It’s designed for patients with node-negative disease with whom you’re
going to be using hormonal therapy, specifically tamoxifen.
DR RAVDIN: I believe the Oncotype DX assay could be used for a patient like
this. It’s designed for patients with node-negative disease with whom you’re
going to be using hormonal therapy, specifically tamoxifen.
The great hope for the Oncotype DX test is that it will be useful in identifying those patients who might benefit in more than an average way from chemotherapy. That story is, I believe, incomplete at this point. They only have data from the NSABP-B-20 adjuvant trial using CMF or M-F (Paik 2006).
![]() DR LOVE: Hal, would you consider obtaining an Oncotype DX recurrence
score for a patient like this?
DR LOVE: Hal, would you consider obtaining an Oncotype DX recurrence
score for a patient like this?
![]() DR BURSTEIN: I love the Oncotype DX test for these kinds of patients because
this is exactly the patient population for whom we all have strongly suspected,
for a long time, that the benefits of chemotherapy are very small. She has a favorable overall prognosis. The tumor is small (less than two centimeters),
and it is estrogen receptor-positive.
DR BURSTEIN: I love the Oncotype DX test for these kinds of patients because
this is exactly the patient population for whom we all have strongly suspected,
for a long time, that the benefits of chemotherapy are very small. She has a favorable overall prognosis. The tumor is small (less than two centimeters),
and it is estrogen receptor-positive.
A number of observations of late have made us question the role of chemotherapy. Retrospective analyses suggest that most of the gains are in hormone receptor-negative disease (Berry 2006). Prospective studies have shown modest benefits under any circumstance. If you look at NSABP-B-20, for which this patient would have been a perfect candidate, you see that the benefits of chemotherapy on top of tamoxifen alone are estimated at only about four percent (Fisher 1997). And most women in that trial had larger tumors with a higher grade than she had.
We know from other work that grade is an important predictor of the benefits of chemotherapy. So this is the situation in which you’re talking about administering chemotherapy for an extremely small benefit, some of which might even be accomplished with ovarian suppression, especially in someone with a hormone receptor-positive, low-grade tumor.
Historically, the dilemma has been that the models of risk or the clinical practice guidelines from the NCCN say, “She has a tumor that is greater than one centimeter. Give her chemotherapy,” yet we all know the gain is small.
The way out of this box is a test like the Oncotype DX assay. We’ve had all this information in front of us for a long time, and yet many of us have lacked the courage of our convictions to say to a woman who has a low-risk tumor, “You don’t need chemotherapy.” That is the crux of the issue.
Tracks 20-21

![]() DR BURSTEIN: Sometimes it’s helpful to look carefully at the lymph nodes
microscopically because the worry about these cases is that they are artifacts
and not biologically significant metastatic disease.
DR BURSTEIN: Sometimes it’s helpful to look carefully at the lymph nodes
microscopically because the worry about these cases is that they are artifacts
and not biologically significant metastatic disease.
Sometimes you can get a feel for that based on how it appears in the lymph node and if other lymph nodes are positive. The first thing I would do is an axillary dissection. If she had extensive residual disease in her other lymph nodes, then I would be far more inclined to consider this breast cancer as opposed to an artifact.
![]() DR NICOLAU: She had an axillary lymph node dissection, and no other lymph
nodes were involved.
DR NICOLAU: She had an axillary lymph node dissection, and no other lymph
nodes were involved.
![]() DR BURSTEIN: Now you’re stuck. You have to decide, in your heart of hearts,
whether you think this is biologically, clinically real cancer in the lymph node.
The current guidelines parse these micrometastatic deposits in various ways:
More than two millimeters is a real involvement, less than two millimeters but
more than 0.2 millimeters is of unknown significance and less than 0.2 millimeters
is considered node-negative (Singletary 2006).
DR BURSTEIN: Now you’re stuck. You have to decide, in your heart of hearts,
whether you think this is biologically, clinically real cancer in the lymph node.
The current guidelines parse these micrometastatic deposits in various ways:
More than two millimeters is a real involvement, less than two millimeters but
more than 0.2 millimeters is of unknown significance and less than 0.2 millimeters
is considered node-negative (Singletary 2006).
She would have a 0.2- to 2.0-mm deposit of unclear significance. The most important treatment for her would be tamoxifen. The second most important might be ovarian suppression. The third and fourth most important might be chemotherapy and/or trastuzumab.
Track 22

![]() DR RAVDIN: A 77-year-old woman will have approximately a 25 percent 10-year risk of competing mortality, even if she’s in good health. However, she
has node-positive, HER2-positive, ER-negative disease, and she has a more
substantial risk of dying of breast cancer than of something else.
DR RAVDIN: A 77-year-old woman will have approximately a 25 percent 10-year risk of competing mortality, even if she’s in good health. However, she
has node-positive, HER2-positive, ER-negative disease, and she has a more
substantial risk of dying of breast cancer than of something else.
This is the type of patient for whom one might seriously consider chemotherapy despite her age. Uncertainties exist about how good chemotherapy is in older patients. However, we have no biological reason to believe it isn’t effective. The temptation arises in what to do in terms of administering trastuzumab if she refuses chemotherapy.
Track 23
![]() DR LOVE: Steve, would you consider trastuzumab without chemotherapy
for this patient?
DR LOVE: Steve, would you consider trastuzumab without chemotherapy
for this patient?
![]() DR JONES: Yes, I would. You’ve indicated she’s terrified of chemotherapy.
She’s also older and much more likely to develop toxicity from chemotherapy.
A year of trastuzumab by itself, however, might be relatively benign. We do
not have data, but we all see the effect of adding trastuzumab to adjuvant
chemotherapy. You could come up with a less-than-standard regimen if you
wanted to and use 12 weeks of weekly paclitaxel, which is fairly nontoxic, in
addition to trastuzumab. I believe you’d probably avoid AC.
DR JONES: Yes, I would. You’ve indicated she’s terrified of chemotherapy.
She’s also older and much more likely to develop toxicity from chemotherapy.
A year of trastuzumab by itself, however, might be relatively benign. We do
not have data, but we all see the effect of adding trastuzumab to adjuvant
chemotherapy. You could come up with a less-than-standard regimen if you
wanted to and use 12 weeks of weekly paclitaxel, which is fairly nontoxic, in
addition to trastuzumab. I believe you’d probably avoid AC.
TC with docetaxel and cyclophosphamide might be an alternative, but an older patient may potentially have a little more toxicity. We will be conducting a pilot safety study of TC with trastuzumab, and I know a few oncologists have used that regimen for patients with lower-risk disease. I believe there are a number of choices, and I’d like to use trastuzumab at the minimum.
![]() DR BURSTEIN: We have planned a multicenter trial, in which patients with
HER2-positive, Stage I breast cancer receive 12 weeks of paclitaxel and trastuzumab
concurrently followed by an additional 40 weeks of trastuzumab. The
goal is to set a relatively low bar — a risk of recurrence of no more than five or
six percent after five years, demonstrating in 300 to 400 patients that you can
achieve a very low risk of recurrence with a reasonably well-tolerated regimen.
DR BURSTEIN: We have planned a multicenter trial, in which patients with
HER2-positive, Stage I breast cancer receive 12 weeks of paclitaxel and trastuzumab
concurrently followed by an additional 40 weeks of trastuzumab. The
goal is to set a relatively low bar — a risk of recurrence of no more than five or
six percent after five years, demonstrating in 300 to 400 patients that you can
achieve a very low risk of recurrence with a reasonably well-tolerated regimen.