
 |
||||||||

| Tracks 1-16 | ||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 3
![]() DR LOVE: Can you describe the background to the release and presentation
of the ECOG-E1199 data?
DR LOVE: Can you describe the background to the release and presentation
of the ECOG-E1199 data?
![]() DR SPARANO: The design called for the data to be released if differences
in the two primary comparisons emerged in any of the Data Monitoring
Committee (DMC) analyses. Initially it was anticipated that full information
would be available after about 1,042 events. The DMC finally elected to
release the data after about 850 events. They felt that with continued follow-up, it was unlikely that a difference would surface, at least with regard to the
primary comparisons.
DR SPARANO: The design called for the data to be released if differences
in the two primary comparisons emerged in any of the Data Monitoring
Committee (DMC) analyses. Initially it was anticipated that full information
would be available after about 1,042 events. The DMC finally elected to
release the data after about 850 events. They felt that with continued follow-up, it was unlikely that a difference would surface, at least with regard to the
primary comparisons.
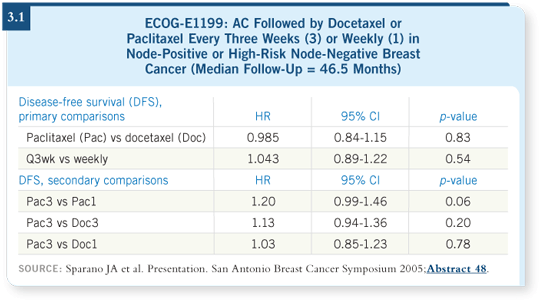
At San Antonio 2005, we presented data that constituted the fourth interim analysis and included 5,052 patients (Sparano 2005). Investigators recorded 856 disease-free survival events, which included relapse, second primary breast cancer or death from other causes. The results indicated no difference whatsoever with regard to the primary comparisons: Paclitaxel versus docetaxel and the every three-week versus weekly schedule (3.1).
However, comparing the individual arms, a difference seemed to emerge for the weekly paclitaxel compared to the every three-week paclitaxel arm. Approximately a 20 percent reduction in the risk of a disease-free survival event was evident in the weekly paclitaxel group, with a p-value of 0.06. No such difference appeared for the other arms — the weekly docetaxel group or the every three-week docetaxel arm compared to the every three-week paclitaxel arm.
![]() DR LOVE: So do you believe that weekly paclitaxel was more favorable in
terms of antitumor effect?
DR LOVE: So do you believe that weekly paclitaxel was more favorable in
terms of antitumor effect?
![]() DR SPARANO: I believe that’s a very reasonable conclusion. Comparing the
every three-week docetaxel arm to every three-week paclitaxel, about a 13
percent reduction in the risk of recurrence is evident with docetaxel, but the
p-value is not statistically significant.
DR SPARANO: I believe that’s a very reasonable conclusion. Comparing the
every three-week docetaxel arm to every three-week paclitaxel, about a 13
percent reduction in the risk of recurrence is evident with docetaxel, but the
p-value is not statistically significant.
Among disease-free survival events, an equivalent number of relapses occurred in the every three-week docetaxel arm compared to the weekly paclitaxel arm. They looked the same, but slightly fewer nonbreast cancer deaths and slightly fewer second primary breast cancers occurred in the every three-week docetaxel arm. The aggregate disease-free survival outcome for the every three-week docetaxel arm was not evaluated as statistically better than the every three-week paclitaxel arm.
Track 4
![]() DR LOVE: Can you discuss side effects and toxicities in E1199?
DR LOVE: Can you discuss side effects and toxicities in E1199?
![]() DR SPARANO: Docetaxel clearly brought more toxicity, whether administered
every three weeks or weekly. With the every three-week schedule, substantially
more neutropenia and infectious complications occurred, as would be
expected from the dose of docetaxel that was used in the trial.
DR SPARANO: Docetaxel clearly brought more toxicity, whether administered
every three weeks or weekly. With the every three-week schedule, substantially
more neutropenia and infectious complications occurred, as would be
expected from the dose of docetaxel that was used in the trial.
![]() DR LOVE: If you were to repeat the trial with preventive growth factors,
would neutropenia be less of an issue?
DR LOVE: If you were to repeat the trial with preventive growth factors,
would neutropenia be less of an issue?
![]() DR SPARANO: No question. Consistent with ASCO guidelines at the time,
colony-stimulating factors were permitted as secondary prophylaxis but not
as primary prophylaxis. In the every three-week docetaxel arm, the dosing
schedule would now be consistent with ASCO guidelines for using a colony-stimulating
factor for primary prophylaxis. In the every three-week docetaxel
arm, we would certainly have seen much less neutropenia — as has been
shown by other trials — if G-CSF had been used as primary prophylaxis.
DR SPARANO: No question. Consistent with ASCO guidelines at the time,
colony-stimulating factors were permitted as secondary prophylaxis but not
as primary prophylaxis. In the every three-week docetaxel arm, the dosing
schedule would now be consistent with ASCO guidelines for using a colony-stimulating
factor for primary prophylaxis. In the every three-week docetaxel
arm, we would certainly have seen much less neutropenia — as has been
shown by other trials — if G-CSF had been used as primary prophylaxis.
For the weekly paclitaxel arm, as one would expect, there was more neuro-sensory toxicity. And for the weekly docetaxel arm, there was tearing and onycholysis.
Tracks 5-6
![]() DR LOVE: What were the conclusions from these data?
DR LOVE: What were the conclusions from these data?
![]() DR SPARANO: The event rate was lower than anticipated, and this was likely
exacerbated by two factors. First, given that a concurrent trial was studying
HER2-positive disease, patients who were at higher risk of relapse and more
likely to benefit from taxane therapy were not included in this study. Second,
the aromatase inhibitors were approved in the adjuvant setting during this
time, which further lowered the event rate.
DR SPARANO: The event rate was lower than anticipated, and this was likely
exacerbated by two factors. First, given that a concurrent trial was studying
HER2-positive disease, patients who were at higher risk of relapse and more
likely to benefit from taxane therapy were not included in this study. Second,
the aromatase inhibitors were approved in the adjuvant setting during this
time, which further lowered the event rate.
![]() DR LOVE: How would you answer a clinician who asks, “What does this
mean to my practice?”
DR LOVE: How would you answer a clinician who asks, “What does this
mean to my practice?”
![]() DR SPARANO: I believe weekly paclitaxel following AC represents another
option for patients who have lymph node-positive breast cancer who may not
be optimal candidates for dose-dense therapy.
DR SPARANO: I believe weekly paclitaxel following AC represents another
option for patients who have lymph node-positive breast cancer who may not
be optimal candidates for dose-dense therapy.
Track 8
![]() DR LOVE: In your practice, what options do you present for 40- to 60-year-old patients in good health with several positive nodes who are not
eligible for a study?
DR LOVE: In your practice, what options do you present for 40- to 60-year-old patients in good health with several positive nodes who are not
eligible for a study?
![]() DR SPARANO: For that type of patient, the usual options that I would discuss would include TAC, dose-dense sequential AC followed by paclitaxel administered
every two weeks or AC followed by weekly paclitaxel. Once I describe
the options, toxicity profiles and duration of therapy, many patients choose the
dose-dense, every two-week approach for eight cycles because of the shorter
duration of therapy and the generally more favorable toxicity profile.
DR SPARANO: For that type of patient, the usual options that I would discuss would include TAC, dose-dense sequential AC followed by paclitaxel administered
every two weeks or AC followed by weekly paclitaxel. Once I describe
the options, toxicity profiles and duration of therapy, many patients choose the
dose-dense, every two-week approach for eight cycles because of the shorter
duration of therapy and the generally more favorable toxicity profile.
![]() DR LOVE: For patients at lower risk, do you ever use dose-dense AC every
two weeks without a taxane?
DR LOVE: For patients at lower risk, do you ever use dose-dense AC every
two weeks without a taxane?
![]() DR SPARANO: Yes I do, even though we don’t have efficacy data to suggest
that this approach is superior to AC administered every three weeks. However,
we do have substantial long-term safety data suggesting that this approach
may be safer in the short term and appears safe in the long term. It is also
completed sooner, so for that reason it is a reasonable approach to the treatment
of a patient at relatively low risk.
DR SPARANO: Yes I do, even though we don’t have efficacy data to suggest
that this approach is superior to AC administered every three weeks. However,
we do have substantial long-term safety data suggesting that this approach
may be safer in the short term and appears safe in the long term. It is also
completed sooner, so for that reason it is a reasonable approach to the treatment
of a patient at relatively low risk.
Track 9
![]() DR LOVE: What are your thoughts on docetaxel/cyclophosphamide (TC)
as an alternative to adjuvant AC?
DR LOVE: What are your thoughts on docetaxel/cyclophosphamide (TC)
as an alternative to adjuvant AC?
![]() DR SPARANO: We now know that TC administered every three weeks is
superior to AC administered every three weeks ( Jones 2005). Therefore, I
believe that represents a reasonable evidence-based option for patients who
have lower-risk early-stage breast cancer. Before the US Oncology study
( Jones 2005), I would commonly use AC administered every two weeks, not
necessarily because I thought it would be more effective — particularly in
estrogen receptor-positive disease — but because the treatment was finished in
a shorter period of time.
DR SPARANO: We now know that TC administered every three weeks is
superior to AC administered every three weeks ( Jones 2005). Therefore, I
believe that represents a reasonable evidence-based option for patients who
have lower-risk early-stage breast cancer. Before the US Oncology study
( Jones 2005), I would commonly use AC administered every two weeks, not
necessarily because I thought it would be more effective — particularly in
estrogen receptor-positive disease — but because the treatment was finished in
a shorter period of time.
![]() DR LOVE: Has your perception of the risk of adjuvant regimens with an
anthracycline, specifically AC, changed in light of recent data on cardiac
toxicity (Shepherd 2006)?
DR LOVE: Has your perception of the risk of adjuvant regimens with an
anthracycline, specifically AC, changed in light of recent data on cardiac
toxicity (Shepherd 2006)?
![]() DR SPARANO: I believe it’s a real complication. We can identify who’s at
high risk, but we can’t identify who will develop a problem with any degree
of certainty. For the patients at high risk, if we have alternatives to AC, then
those options should be seriously considered.
DR SPARANO: I believe it’s a real complication. We can identify who’s at
high risk, but we can’t identify who will develop a problem with any degree
of certainty. For the patients at high risk, if we have alternatives to AC, then
those options should be seriously considered.
Tracks 12-13
![]() DR LOVE: Can you discuss the background of the TAILORx study?
DR LOVE: Can you discuss the background of the TAILORx study?
![]() DR SPARANO: This trial had its genesis in a program at NCI called PACCT
(Program for the Assessment of Clinical Cancer Tests), which was designed
to meet the challenge of integrating molecular proteomic and epigenomic
markers into clinical practice and clinical decision-making. Of course, many
molecular markers are available, and a variety of cancer types would be eligible
for refining what patient subgroups are benefiting from specific treatments.
DR SPARANO: This trial had its genesis in a program at NCI called PACCT
(Program for the Assessment of Clinical Cancer Tests), which was designed
to meet the challenge of integrating molecular proteomic and epigenomic
markers into clinical practice and clinical decision-making. Of course, many
molecular markers are available, and a variety of cancer types would be eligible
for refining what patient subgroups are benefiting from specific treatments.
The time and the data seem to be right for targeting ER-positive, lymph node-negative breast cancer and for using the Oncotype DX test as part of that evaluation for several reasons. First, ER-positive, lymph node-negative breast cancer accounts for about half of all breast cancer diagnosed in North America each year. Second, the majority of these patients would be considered eligible for receiving chemotherapy based on current practice guidelines, such as the NCCN and St Gallen guidelines.
However, we know that 80 to 85 percent of these patients would be adequately treated with endocrine therapy alone and that the absolute benefit from chemotherapy is in the range of three to five percent. We’re obviously overtreating the great majority of the patients whom we treat with adjuvant chemotherapy.
The question was whether we could use a molecular diagnostic test to provide a clear treatment path for patients who didn’t require adjuvant chemotherapy and one for those who did. The trial was also an opportunity to refine the utility of the test and determine whether other subsets of patients were deriving benefit from chemotherapy. Because TAILORx (Trial Assigning Individualized Options for Treatment) was the first trial to emanate from the PACCT program, it has also been called the PACCT-1 trial.
At the time that TAILORx was being designed, a handful of externally validated molecular tests were available. The Oncotype DX was chosen to be integrated into this trial because, first, it was validated for patients with ER-positive, node-negative disease, for whom we believed integrating a molecular test would be most beneficial.
Second, the test can be performed on routinely processed paraffin-embedded tissue, a huge advantage compared to some of the other molecular diagnostic tests. Third, the work that led to the CLIA (Clinical Laboratory Improvement Amendments) approval of this test was done through the Cooperative Group system, building on the work done by the Cooperative Groups and the Intergroup (Paik 2003).
From a panel of about 250 genes believed likely to be clinically relevant, the scientists at Genomic Health evaluated three data sets that included patients with a mixture of clinical features: node-positive, node-negative, HER2-positive, HER2-negative, ER-positive and ER-negative disease.
They honed these down to 16 genes that cluster into various groups: an ER group, a proliferation group, a HER2 group and others. With that information, they developed the algorithm that was used to develop the recurrence score.
The algorithm was prospectively validated in the NSABP-B-14 trial for patients who had ER-positive, lymph node-negative disease (Paik 2003). The recurrence score predicted outcome, whether evaluated as a trinary variable with low, intermediate or high risk or as a continuous variable. In the NSABP-B-20 trial, which compares tamoxifen to tamoxifen with CMF, only patients who had a high recurrence score seem to be benefiting from the administration of CMF (Paik 2004).
![]() DR LOVE: Do you believe that this assay should be utilized in clinical practice?
DR LOVE: Do you believe that this assay should be utilized in clinical practice?
![]() DR SPARANO: Absolutely. I believe that this assay can and should be used in
the clinical decision-making process if the result of the test is likely to influence
a treatment decision.
DR SPARANO: Absolutely. I believe that this assay can and should be used in
the clinical decision-making process if the result of the test is likely to influence
a treatment decision.
For example, if you have a patient for whom endocrine therapy alone is the only option, the test can provide more precise prognostic information, but it’s not going to alter your management. I would use the test in circumstances in which the results could potentially alter management.
Tracks 14-15
![]() DR LOVE: Would you describe the TAILORx study design (see page 4
[1])?
DR LOVE: Would you describe the TAILORx study design (see page 4
[1])?
![]() DR SPARANO: The study is targeting patients up to age 75 who have ER-positive,
axillary lymph node-negative breast cancer. The tumor must be at
least 1.1 centimeters in size, or it could be between six millimeters and 11
millimeters if unfavorable histologic features are present, such as intermediate
or poor nuclear grade or lymphovascular invasion. Patients must also have
HER2-negative disease, determined by their local laboratory and defined as
either FISH-negative or 0 to 1+ by the DAKO HercepTest™ or another FDA-approved
test.
DR SPARANO: The study is targeting patients up to age 75 who have ER-positive,
axillary lymph node-negative breast cancer. The tumor must be at
least 1.1 centimeters in size, or it could be between six millimeters and 11
millimeters if unfavorable histologic features are present, such as intermediate
or poor nuclear grade or lymphovascular invasion. Patients must also have
HER2-negative disease, determined by their local laboratory and defined as
either FISH-negative or 0 to 1+ by the DAKO HercepTest™ or another FDA-approved
test.
Given that the study will evaluate 10-year outcomes, patients must have a life expectancy of at least 10 years, making those with significant comorbid medical conditions ineligible. Of course, patients need to be acceptable candidates for chemotherapy, and they need to agree in principle to receive chemotherapy.
![]() DR LOVE: Can patients receive any type of chemotherapy, or is it specified?
DR LOVE: Can patients receive any type of chemotherapy, or is it specified?
![]() DR SPARANO: Physicians can choose from a range of standard chemotherapy
regimens in the protocol. Generally these are consistent with NCCN guidelines
and also now include docetaxel/cyclophosphamide as an option ( Jones
2005). Patients can enroll on another CTSU trial, provided it is consistent
with TAILORx-assigned treatment.
DR SPARANO: Physicians can choose from a range of standard chemotherapy
regimens in the protocol. Generally these are consistent with NCCN guidelines
and also now include docetaxel/cyclophosphamide as an option ( Jones
2005). Patients can enroll on another CTSU trial, provided it is consistent
with TAILORx-assigned treatment.
![]() DR LOVE: Patients at lower risk receive only hormone therapy according to
physician choice. The focus, then, is the patients at intermediate risk, who are
randomly assigned to chemotherapy or not?
DR LOVE: Patients at lower risk receive only hormone therapy according to
physician choice. The focus, then, is the patients at intermediate risk, who are
randomly assigned to chemotherapy or not?
![]() DR SPARANO: Yes. This is defined a little differently than in the original
descriptions by Genomic Health and NSABP. We’re targeting patients with
a recurrence score of 11-25, who will be randomly assigned to either chemotherapy
and hormonal therapy, which is considered the standard treatment
arm, or hormonal therapy alone, which is considered the experimental arm.
It is designed as a noninferiority trial, powered to detect a three percent or greater reduction in disease-free survival by the omission of chemotherapy.
DR SPARANO: Yes. This is defined a little differently than in the original
descriptions by Genomic Health and NSABP. We’re targeting patients with
a recurrence score of 11-25, who will be randomly assigned to either chemotherapy
and hormonal therapy, which is considered the standard treatment
arm, or hormonal therapy alone, which is considered the experimental arm.
It is designed as a noninferiority trial, powered to detect a three percent or greater reduction in disease-free survival by the omission of chemotherapy.
![]() DR LOVE: Why was 11 to 25 chosen for the midrange recurrence score?
DR LOVE: Why was 11 to 25 chosen for the midrange recurrence score?
![]() DR SPARANO: First, at the upper end, we dialed down from 31 to 25 because
we wanted to minimize the potential for undertreating patients in the upper
range. In fact, when the NSABP reanalyzed the data, the treatment effect of
chemotherapy in patients with a recurrence score of 31 or higher was similar
to that in patients who had a recurrence score of 26 or higher.
DR SPARANO: First, at the upper end, we dialed down from 31 to 25 because
we wanted to minimize the potential for undertreating patients in the upper
range. In fact, when the NSABP reanalyzed the data, the treatment effect of
chemotherapy in patients with a recurrence score of 31 or higher was similar
to that in patients who had a recurrence score of 26 or higher.
Second, at the lower end of the range, a recurrence score of 11 was chosen because, with that score, the risk of both distant and local recurrence is in the range of five to 10 percent, and that’s the threshold at which we typically recommend chemotherapy.
Third, the B-20 trial assessed the risk of recurrence as a continuous variable for patients treated with tamoxifen or tamoxifen with chemotherapy. In that trial, the curves begin to separate at about 10 or 11, and by about 25 they are quite divergent (Paik 2006).
Yet the 95 percent confidence intervals in that range of 11 to 25 completely overlap. These are the principal reasons why we chose to use a different range of recurrence scores than were originally reported.