
 |
||||||||

| Tracks 1-12 | ||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 3-4
![]() DR LOVE: Can you discuss your work on NSABP trial B-31 evaluating
tissue predictors of outcome in HER2-positive disease?
DR LOVE: Can you discuss your work on NSABP trial B-31 evaluating
tissue predictors of outcome in HER2-positive disease?
![]() DR PAIK: We are interested in learning whether we can identify prognostic
factors or predictive markers for benefit from trastuzumab. Although patients
in NSABP-B-31 derived a significant benefit from trastuzumab, their four-year
recurrence rate was still about 15 percent, so they were not completely cured.
DR PAIK: We are interested in learning whether we can identify prognostic
factors or predictive markers for benefit from trastuzumab. Although patients
in NSABP-B-31 derived a significant benefit from trastuzumab, their four-year
recurrence rate was still about 15 percent, so they were not completely cured.
We had to design a next-generation trial in which we could add more agents, such as bevacizumab, but we didn’t want to administer additional treatment to everybody, so we had to generate the prognostic model for trastuzumab-treated patients. We were trying to uncover many markers.
![]() DR LOVE: What specific markers are you evaluating?
DR LOVE: What specific markers are you evaluating?
![]() DR PAIK: Obvious candidates such as HER2 gene copy or expression level
by immunohistochemistry in addition to some other molecules, such as PTEN
(Fujita 2006), are described as important in a HER2 receptor signaling pathway.
DR PAIK: Obvious candidates such as HER2 gene copy or expression level
by immunohistochemistry in addition to some other molecules, such as PTEN
(Fujita 2006), are described as important in a HER2 receptor signaling pathway.
![]() DR LOVE: What have you learned about gene copy number and response to
trastuzumab?
DR LOVE: What have you learned about gene copy number and response to
trastuzumab?
![]() DR PAIK: That’s interesting biology. As trastuzumab is targeted to HER2,
one would expect that the HER2 level should be a direct predictor of degree
of benefit from trastuzumab in the adjuvant setting. But when we assessed
the degree of benefit by HER2 gene amplification level, it was not directly
predictive (Shak 2006). Because of the way eligibility was set up in the B-31
trial, about 10 percent of the patients overall turned out to have a normal gene
copy number — no amplification.
DR PAIK: That’s interesting biology. As trastuzumab is targeted to HER2,
one would expect that the HER2 level should be a direct predictor of degree
of benefit from trastuzumab in the adjuvant setting. But when we assessed
the degree of benefit by HER2 gene amplification level, it was not directly
predictive (Shak 2006). Because of the way eligibility was set up in the B-31
trial, about 10 percent of the patients overall turned out to have a normal gene
copy number — no amplification.
![]() DR LOVE: In other words, 10 percent of the patients essentially had HER2-negative disease? Were there enough patients or events to document any
impact of trastuzumab on HER2-negative disease?
DR LOVE: In other words, 10 percent of the patients essentially had HER2-negative disease? Were there enough patients or events to document any
impact of trastuzumab on HER2-negative disease?
![]() DR PAIK: It’s a small number. In that small number of patients with normal-copy
HER2, a trend to benefit did appear. That was surprising, and when we
conducted an interaction test or a test for impact of benefit across all categories
of gene amplification, we didn’t find any significance. So it appears as though
the HER2 gene copy is not a good predictor of degree of benefit.
DR PAIK: It’s a small number. In that small number of patients with normal-copy
HER2, a trend to benefit did appear. That was surprising, and when we
conducted an interaction test or a test for impact of benefit across all categories
of gene amplification, we didn’t find any significance. So it appears as though
the HER2 gene copy is not a good predictor of degree of benefit.
![]() DR LOVE: Can you discuss the new ASCO/CAP guideline for HER2 testing?
DR LOVE: Can you discuss the new ASCO/CAP guideline for HER2 testing?
![]() DR PAIK: The new guideline adopted by ASCO and CAP (Wolff 2007),
published in the Journal of Clinical Oncology, states that if the FISH ratio is
between 1.8 and 2.2, a result that would be regarded as equivocal, patients
should be tested again or tested with HER2 IHC.
DR PAIK: The new guideline adopted by ASCO and CAP (Wolff 2007),
published in the Journal of Clinical Oncology, states that if the FISH ratio is
between 1.8 and 2.2, a result that would be regarded as equivocal, patients
should be tested again or tested with HER2 IHC.
![]() DR LOVE: What else that was new came out in that guideline?
DR LOVE: What else that was new came out in that guideline?
![]() DR PAIK: That is the bottom line — essentially it recommends strict quality
control for the laboratories that conduct testing for HER2.
DR PAIK: That is the bottom line — essentially it recommends strict quality
control for the laboratories that conduct testing for HER2.
Track 5
![]() DR LOVE: Do you think the situation with ER testing is any better than
that with HER2?
DR LOVE: Do you think the situation with ER testing is any better than
that with HER2?
![]() DR PAIK: No, ER testing I believe is much worse off.
DR PAIK: No, ER testing I believe is much worse off.
![]() DR LOVE: Do you see CAP, ASCO and other major entities focusing on ER
testing?
DR LOVE: Do you see CAP, ASCO and other major entities focusing on ER
testing?
![]() DR PAIK: Yes. I do believe the next target will be ER assay standardization.
For HER2, we had two competing assays that we could always use to
compare data, and besides, the expression level of HER2 is somewhat bimodal
in distribution because of amplification. The ER is not like that — it’s more
continuous.
DR PAIK: Yes. I do believe the next target will be ER assay standardization.
For HER2, we had two competing assays that we could always use to
compare data, and besides, the expression level of HER2 is somewhat bimodal
in distribution because of amplification. The ER is not like that — it’s more
continuous.
The current-generation immunohistochemical assay that pathology labs use incorporates an amplification method for the signal to generate color in the slide, and this biases the assay as too sensitive at the low level of estrogen receptor. So it will be a fairly difficult effort.
Tracks 7-8
![]() DR LOVE: Can you review the data on cMYC in the B-31 adjuvant
trastuzumab study?
DR LOVE: Can you review the data on cMYC in the B-31 adjuvant
trastuzumab study?
![]() DR PAIK: A benefit in relapse rate was still evident among the patients with
cMYC-nonamplified disease, but it was much attenuated. Analysis of the
interaction between cMYC amplification and benefit from trastuzumab (Kim
2005) demonstrates an extremely strong p-value (p < 0.001). I wondered
why cMYC turned out to be the predictor. It turns out that I was relatively
uninformed about cMYC biology.
DR PAIK: A benefit in relapse rate was still evident among the patients with
cMYC-nonamplified disease, but it was much attenuated. Analysis of the
interaction between cMYC amplification and benefit from trastuzumab (Kim
2005) demonstrates an extremely strong p-value (p < 0.001). I wondered
why cMYC turned out to be the predictor. It turns out that I was relatively
uninformed about cMYC biology.
When I began to read the literature about cMYC, I realized that our results make a lot of sense. The cMYC gene is a transcription factor, and it essentially regulates a lot of genes, including those relating to cell proliferation and also cell death. It’s crazy that the same molecule regulates both cell proliferation and cell death — it’s almost like an inherent biological defense the cells have against cancer.
The genes that are important in the body are always dual regulators, and cMYC is one of them. Because it has this dual capability of inducing both proliferation and cell death, when this molecule becomes abnormal (deregulated expression due to gene amplification or mutation and so on) the cells go through cell death, so they cannot become cancer cells.
The only way cancer cells can develop is when they can bypass survivor factors that inhibit the apoptotic signal.
My theory is that HER2 is one of the survivor factors. In those cells that have gene amplification of both HER2 and cMYC, which is a small subset of breast cancer patients (about six percent), HER2 is not the oncogene in that situation — cMYC is the oncogene. HER2 helps cMYC to become the oncogene by suppressing the cell death signal. In that setting, because of the high survivor signal coming from HER2, the cells are inherent and resistant to chemotherapy.
![]() DR LOVE: So chemotherapy is part of the equation here too?
DR LOVE: So chemotherapy is part of the equation here too?
![]() DR PAIK: Right. If you administer trastuzumab in this situation, the survivor
signal coming from HER2 goes away, and it leaves the strong cMYC to
induce cell death, especially in combination with chemotherapy, and causes a
massive cell suicide.
DR PAIK: Right. If you administer trastuzumab in this situation, the survivor
signal coming from HER2 goes away, and it leaves the strong cMYC to
induce cell death, especially in combination with chemotherapy, and causes a
massive cell suicide.
![]() DR LOVE: That’s interesting. We’ve always had this question about how much
of the effect of trastuzumab is related to synergy with chemotherapy. We
know as a single agent trastuzumab has significant activity. Does your model
make sense in terms of the effect of trastuzumab without chemotherapy?
DR LOVE: That’s interesting. We’ve always had this question about how much
of the effect of trastuzumab is related to synergy with chemotherapy. We
know as a single agent trastuzumab has significant activity. Does your model
make sense in terms of the effect of trastuzumab without chemotherapy?
![]() DR PAIK: We know of two possible ways to interpret these data. One interpretation
is that the trastuzumab obviously causes immune-related cell death,
too, so the reason that cMYC-negative patients also gain some benefit in
disease-free survival might be the fact that immune killing occurs in addition
to the chemotherapy killing.
DR PAIK: We know of two possible ways to interpret these data. One interpretation
is that the trastuzumab obviously causes immune-related cell death,
too, so the reason that cMYC-negative patients also gain some benefit in
disease-free survival might be the fact that immune killing occurs in addition
to the chemotherapy killing.
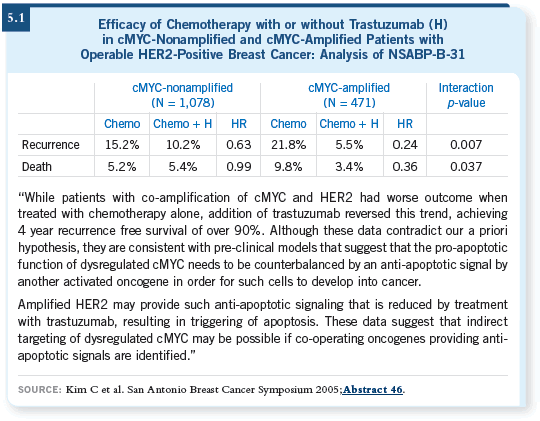
The other possibility is that in the cMYC-nonamplified group, trastuzumab is simply causing growth inhibition, not cell death. That’s why you see a continued failure even on trastuzumab if you view the Kaplan-Meier plot in the cMYC-nonamplified group, but if you evaluate the cMYC-amplified cohort, you see almost no failure after two years (5.1).
So for the patient with cMYC amplification, the mechanism of trastuzumab varies mainly with chemotherapy, and apoptosis occurs. In cMYC-negative disease, trastuzumab simply inhibits growth.
Using that hypothesis, the prediction is that for cMYC-positive disease, you might not have to administer trastuzumab for one year. Perhaps you only need to administer it together with chemotherapy. For patients with cMYC-negative disease, you have to administer trastuzumab essentially forever.
Track 9
![]() DR LOVE: Can you update us on the status of your research on Oncotype DX?
DR LOVE: Can you update us on the status of your research on Oncotype DX?
![]() DR PAIK: Two interesting issues were raised by many people regarding the
Oncotype DX assay. Our data showed that if patients had HER2 amplification,
they were usually categorized as being at high or intermediate risk and none
of them were at low risk.
DR PAIK: Two interesting issues were raised by many people regarding the
Oncotype DX assay. Our data showed that if patients had HER2 amplification,
they were usually categorized as being at high or intermediate risk and none
of them were at low risk.
Steve Shak has screened approximately 10,000 patients so far and has found some patients have HER2 amplification but are still at low risk (Shak 2006). So he believes they still must be tested, but my bias considering the NSABPB-14 data is that they don’t need to be tested.
Because of those data, some people are arguing that if you take out the patients with HER2 amplification, then the Oncotype DX assay will not be as strong a prognosticator for patients with HER2-negative disease. We did assess the HER2-negative subset in B-14, and it worked exactly as it did for the overall cohort.
The other issue is that everybody wants to find out whether the Oncotype DX assay can be used for patients with node-positive disease. For that, we are eagerly waiting for Kathy Albain’s SWOG study (SWOG-S8814A-ICSC) to learn whether it is also predictive of benefits of chemotherapy for patients with node-positive disease.