
 |
||||||||

| Tracks 1-16 | ||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Can you discuss the BCIRG 006 trial results that were updated
at the 2006 San Antonio Breast Cancer Symposium?
DR LOVE: Can you discuss the BCIRG 006 trial results that were updated
at the 2006 San Antonio Breast Cancer Symposium?
![]() DR WINER: Dr Slamon presented the second analysis of BCIRG 006. Once
again, it was clear that the use of adjuvant trastuzumab improved outcomes
compared to no trastuzumab. Many people thought the big news was the
update on the TCH regimen. The trial compared three treatment regimens in
women with HER2-positive breast cancer as defined by FISH (Slamon 2006).
DR WINER: Dr Slamon presented the second analysis of BCIRG 006. Once
again, it was clear that the use of adjuvant trastuzumab improved outcomes
compared to no trastuzumab. Many people thought the big news was the
update on the TCH regimen. The trial compared three treatment regimens in
women with HER2-positive breast cancer as defined by FISH (Slamon 2006).
One group of women received four cycles of AC followed by four cycles of docetaxel. The second group of women received four cycles of AC followed by four cycles of docetaxel administered concurrently with trastuzumab and then followed by the completion of one year of trastuzumab. The third arm was the TCH regimen, which was docetaxel, carboplatin and trastuzumab (Slamon 2006).
In the TCH arm, no anthracycline was used and trastuzumab was administered from the beginning of the chemotherapy. The other two arms included an anthracycline, which did not allow for the concurrent administration of trastuzumab from the start (Slamon 2006).
When these data were initially presented at the 2005 San Antonio Breast Cancer Symposium, the addition of trastuzumab was found to improve disease-free survival. A suggestion emerged that the patients receiving AC followed by docetaxel/trastuzumab seemed to do better than those receiving the nonanthracycline-containing regimen. The differences were not statistically significant, but the suggestion was that more recurrences occurred in the TCH arm (Slamon 2005).
I believe that led many people to feel cautious about using TCH other than for the patient who had a contraindication to the use of an anthracycline. However, in the December update, the two trastuzumab-containing arms appeared to behave similarly. Both of the trastuzumab-containing arms recorded fewer recurrences than the nontrastuzumab-containing arm, and no dramatic difference seemed evident (Slamon 2006).
Two perspectives on these data are possible. One would be, “This is great, and we don’t have to use an anthracycline.” The other would be, “These are encouraging data, and they are a sign that perhaps we will be able to eliminate the anthracycline. At the same time, maybe it’s best not to forget that in all the other adjuvant trastuzumab studies an anthracycline was used, and we still have a lot more experience with anthracycline- than nonanthracycline-containing regimens.”
Maybe it’s not time to throw out the anthracycline yet, although it certainly gives us courage to examine that issue further. I am in that second camp. In my practice, for most patients I would continue to use an anthracycline followed by a taxane and trastuzumab. I am, however, a little more comfortable than I was a year ago in skipping the anthracycline if a patient has a reason not to receive it.
Track 2
![]() DR LOVE: Can you review the data from BCIRG 006 evaluating TOPO
II amplification?
DR LOVE: Can you review the data from BCIRG 006 evaluating TOPO
II amplification?
![]() DR WINER: It is worth bearing in mind that these are subset analyses, and at
our center we don’t perform TOPO II testing. In my view, this is not ready
for prime time.
DR WINER: It is worth bearing in mind that these are subset analyses, and at
our center we don’t perform TOPO II testing. In my view, this is not ready
for prime time.
A year ago, the suggestion emerged from 006 that for those women with TOPO II amplification in addition to HER2 amplification, the anthracycline seemed to matter more. The women who received AC followed by docetaxel/ trastuzumab and had TOPO II amplification had the best outcome, and women who received TCH in the presence of TOPO II amplification perhaps didn’t do quite as well (Slamon 2005).
This year, Dr Slamon presented two findings. First, in general, women whose
tumors were TOPO II and HER2 amplified seemed to have a better outcome
than those whose tumors were not TOPO II amplified. Second, among those
women whose tumors were TOPO II amplified, a difference didn’t seem to
appear between TCH and AC![]() TH. It is also worth pointing out that among
those women whose tumors were TOPO II amplified, those who didn’t
receive trastuzumab also did quite well (Slamon 2006).
TH. It is also worth pointing out that among
those women whose tumors were TOPO II amplified, those who didn’t
receive trastuzumab also did quite well (Slamon 2006).
Track 8
![]() DR LOVE: How do you approach patients with small (<1 cm), HER2-positive, node-negative disease?
DR LOVE: How do you approach patients with small (<1 cm), HER2-positive, node-negative disease?
![]() DR WINER: For a woman with a 9-mm, ER-negative, node-negative, HER2-positive tumor, a number of people — and I am one of them — would at least
consider using trastuzumab.
DR WINER: For a woman with a 9-mm, ER-negative, node-negative, HER2-positive tumor, a number of people — and I am one of them — would at least
consider using trastuzumab.
I would also be more inclined to consider a nonanthracycline-containing regimen. In fact, we are developing a large Phase II trial evaluating weekly paclitaxel with trastuzumab for these patients. The trial will simply try to achieve a recurrence rate below a certain percentage.
If we achieve that rate, we will consider it a success. A success could either mean we would go on to another study, which I believe is unlikely, or we would feel comfortable with the regimen.
Track 10
![]() DR LOVE: Where are we with the adjuvant trials evaluating bevacizumab?
DR LOVE: Where are we with the adjuvant trials evaluating bevacizumab?
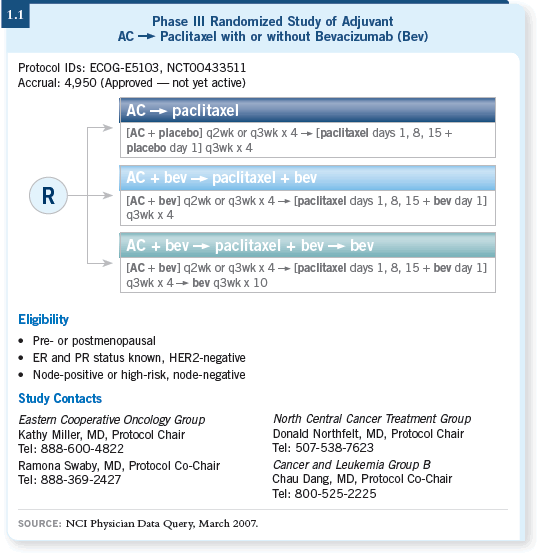
![]() DR WINER: Within the Intergroup, we have all endorsed a study (ECOG-E5103)
comparing AC followed by paclitaxel/bevacizumab to AC followed by
paclitaxel alone. That study will also have a randomization between six and 12
months of bevacizumab (1.1).
DR WINER: Within the Intergroup, we have all endorsed a study (ECOG-E5103)
comparing AC followed by paclitaxel/bevacizumab to AC followed by
paclitaxel alone. That study will also have a randomization between six and 12
months of bevacizumab (1.1).
![]() DR LOVE: Are you allowing dose-dense AC
DR LOVE: Are you allowing dose-dense AC![]() paclitaxel in that study?
paclitaxel in that study?
![]() DR WINER: That decision is left to the physician. We in CALGB pushed hard
for dose-dense
DR WINER: That decision is left to the physician. We in CALGB pushed hard
for dose-dense
AC![]() paclitaxel, so there is flexibility about how the AC is
administered.
paclitaxel, so there is flexibility about how the AC is
administered.
![]() DR LOVE: What do we know from the pilot studies that have been
conducted?
DR LOVE: What do we know from the pilot studies that have been
conducted?
![]() DR WINER: One pilot study conducted by ECOG (E2104) had two arms:
(1) bevacizumab administered concurrently with AC and paclitaxel or (2) AC
followed by bevacizumab administered concurrently with paclitaxel only. To
my knowledge, we do not have any data yet, but we will before the other
study starts. It will be important to make sure that administering bevacizumab
during the AC is acceptable from a cardiac toxicity standpoint.
DR WINER: One pilot study conducted by ECOG (E2104) had two arms:
(1) bevacizumab administered concurrently with AC and paclitaxel or (2) AC
followed by bevacizumab administered concurrently with paclitaxel only. To
my knowledge, we do not have any data yet, but we will before the other
study starts. It will be important to make sure that administering bevacizumab
during the AC is acceptable from a cardiac toxicity standpoint.
![]() DR LOVE: Do we know anything about cardiac safety?
DR LOVE: Do we know anything about cardiac safety?
![]() DR WINER: Concern has arisen about cardiac safety with bevacizumab. The
number of events is by no means large, but a handful of cases of symptomatic
cardiac toxicity have been reported with the use of bevacizumab, typically in
conjunction with chemotherapy, although not with an anthracycline.
DR WINER: Concern has arisen about cardiac safety with bevacizumab. The
number of events is by no means large, but a handful of cases of symptomatic
cardiac toxicity have been reported with the use of bevacizumab, typically in
conjunction with chemotherapy, although not with an anthracycline.
Also, a poster presentation by Mark Pegram at the 2006 San Antonio Breast Cancer Symposium evaluated trastuzumab and bevacizumab, which is a different issue because it involved combining the two antibodies. Some suggestion of cardiac toxicity appeared (Pegram 2006), although how much of it was caused by trastuzumab versus bevacizumab versus the combination is difficult to say.
We know bevacizumab causes hypertension in some women, and hypertension puts an added strain on the heart. It is not inconceivable that a problem could exist for at least a small number of women.
Track 13
![]() DR LOVE: What do you recommend for the patient who received an
adjuvant taxane less than one year ago and now experiences a relapse?
DR LOVE: What do you recommend for the patient who received an
adjuvant taxane less than one year ago and now experiences a relapse?
![]() DR WINER: I struggle with what to do with that patient. Fortunately, it
doesn’t come up too often. Those types of patients were excluded from
ECOG-E2100. About 20 percent of the patients in ECOG-E2100 had
received a prior taxane but not in the past year (Miller 2005).
DR WINER: I struggle with what to do with that patient. Fortunately, it
doesn’t come up too often. Those types of patients were excluded from
ECOG-E2100. About 20 percent of the patients in ECOG-E2100 had
received a prior taxane but not in the past year (Miller 2005).
I am unenthusiastic about using a taxane again for that patient. So that would be a setting in which I would still want to use bevacizumab. I would use it either with capecitabine or vinorelbine. We conducted a Phase II trial with vinorelbine demonstrating that it was safe and reasonably effective (Burstein 2002), but I still don’t believe we know what to do with these patients.