
 |
||||||||

| Tracks 1-17 | ||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Can you comment on the new assay targeting intrinsic breast
cancer subtypes, which you were involved in developing?
DR LOVE: Can you comment on the new assay targeting intrinsic breast
cancer subtypes, which you were involved in developing?
![]() DR ELLIS: We’ve developed an assay based on 50 genes that generate the
intrinsic subtypes — luminal A, luminal B, HER2-enriched and basal-like
(Parker 2008). The fifth class, called normal-like, is not actually a tumor type.
DR ELLIS: We’ve developed an assay based on 50 genes that generate the
intrinsic subtypes — luminal A, luminal B, HER2-enriched and basal-like
(Parker 2008). The fifth class, called normal-like, is not actually a tumor type.
Rather, it means that the sample is too tumor sparse to identify a subtype.
I believe that this assay will produce a gold standard for naming the intrinsic subtypes of breast cancer, which is important as we move forward and begin designing subtype-specific trials.
Tracks 2-3
![]() DR ELLIS: Another area of my research is the issue of predicting benefit
from endocrine therapy. The big conundrum with hormone receptor-positive
disease is that some of these tumors are biologically hormone dependent while
others are hormone independent. We haven’t had a good way of sorting these
two groups, so we evaluated what could be considered an in vivo estrogen
dependence test simply based on the labeling index of the tumor before and
after starting an aromatase inhibitor or tamoxifen.
DR ELLIS: Another area of my research is the issue of predicting benefit
from endocrine therapy. The big conundrum with hormone receptor-positive
disease is that some of these tumors are biologically hormone dependent while
others are hormone independent. We haven’t had a good way of sorting these
two groups, so we evaluated what could be considered an in vivo estrogen
dependence test simply based on the labeling index of the tumor before and
after starting an aromatase inhibitor or tamoxifen.
Mitch Dowsett and I created a relapse score or relapse model that accurately identifies groups of patients who have approximately a 100 percent relapse-free survival at five to seven years. Their tumors are characterized by low stage and low labeling index after the start of endocrine therapy and, interestingly, the maintenance of estrogen receptor in the tumor. Losing estrogen receptor was found to be an independent bad prognostic factor.
Historically, we’ve been examining baseline tumors and then trying to predict benefit from endocrine therapy. However, I believe that we need to shift the paradigm and profile the tumor after two to four weeks of treatment. When we do that, we obtain a much better prognostic index because while the baseline sample will predict outcomes in the absence of therapy, what we want is to predict outcomes in the presence of endocrine therapy.
For example, any predictive multigene model that includes proliferation markers and estrogen-dependent genes should work much better to predict the outcomes of endocrine therapy in samples taken after starting treatment because those transcriptional signatures will be affected by therapy. Patients in whom the transcriptional signature for proliferation is switched off should fare better than those patients in whom it does not switch off.
When we used our assay in the neoadjuvant setting, we found exactly that. In patients whose tumors had a high-risk profile at baseline because they had a proliferation signature, if that signature was turned off, then those patients fared well in the long term. If the proliferation signature was not switched off by endocrine therapy, then those patients fared poorly and, in fact, those tumors were associated with ER loss at the end of the four months of neoadjuvant endocrine therapy (Ellis 2008).
Track 7
![]() DR LOVE: What do you think about the data presented at ASCO by Pam
Goodwin on vitamin D levels and cancer recurrence
DR LOVE: What do you think about the data presented at ASCO by Pam
Goodwin on vitamin D levels and cancer recurrence
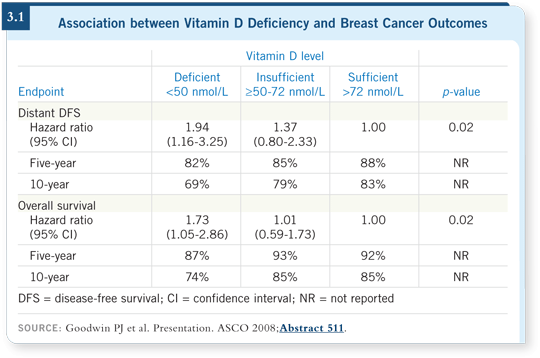
![]() DR ELLIS: The Canadian Clinical Trials Group presented data at ASCO on
vitamin D levels and their effect on breast cancer outcomes (Goodwin 2008;
[3.1]). It’s a little complicated, but the message was that patients with extreme
vitamin D deficiencies seemed to have worse relapse-free survival rates. This is
consistent with other data suggesting that a low vitamin D level may be associated
with a higher breast cancer incidence.
DR ELLIS: The Canadian Clinical Trials Group presented data at ASCO on
vitamin D levels and their effect on breast cancer outcomes (Goodwin 2008;
[3.1]). It’s a little complicated, but the message was that patients with extreme
vitamin D deficiencies seemed to have worse relapse-free survival rates. This is
consistent with other data suggesting that a low vitamin D level may be associated
with a higher breast cancer incidence.
I believe that we need to conduct more detailed studies, and a number of studies have already addressed diet, exercise and relapse-free survival. Women may be able to take several steps to improve their outcomes, such as being physically active, taking adequate vitamin D and maintaining a relatively low body mass index.
I measure the vitamin D level in every breast cancer patient I see, and if the result is in the osteomalacia range, I administer 50,000 units weekly for three to six months. It’s frightening how much severe vitamin D deficiency one sees in a breast cancer population.
Track 8
![]() DR LOVE: What was your reaction to the ASCO data from Joyce
O’Shaughnessy’s trial evaluating lapatinib with or without trastuzumab
(O’Shaughnessy 2008)?
DR LOVE: What was your reaction to the ASCO data from Joyce
O’Shaughnessy’s trial evaluating lapatinib with or without trastuzumab
(O’Shaughnessy 2008)?
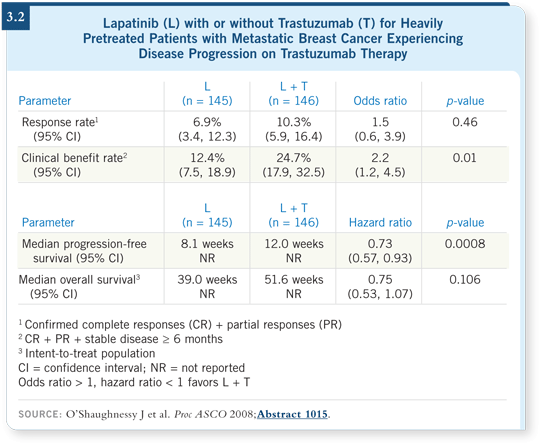
![]() DR ELLIS: In this modest-sized Phase III trial, patients with trastuzumab-resistant
metastatic breast cancer were, upon disease progression, randomly
assigned to lapatinib alone or the reintroduction of trastuzumab with lapatinib.
DR ELLIS: In this modest-sized Phase III trial, patients with trastuzumab-resistant
metastatic breast cancer were, upon disease progression, randomly
assigned to lapatinib alone or the reintroduction of trastuzumab with lapatinib.
The data showed that the combination seemed relatively safe, and the number of patients with progression-free disease at six months increased from 13 percent with lapatinib alone to 28 percent with trastuzumab/lapatinib (O’Shaughnessy 2008; [3.2]).
That seems to suggest that in the resistance setting, continuing trastuzumab and adding lapatinib is a better strategy than stopping trastuzumab and replacing it with lapatinib.
While a case is beginning to be built for the combination of trastuzumab and lapatinib without chemotherapy, I certainly would not recommend that as standard at this point. We don’t have enough data, and we need to confirm it.
Tracks 12-13
![]() DR LOVE: How do you feel about the data from the aTTom and ATLAS
trials, both evaluating 10 versus five years of adjuvant tamoxifen?
DR LOVE: How do you feel about the data from the aTTom and ATLAS
trials, both evaluating 10 versus five years of adjuvant tamoxifen?
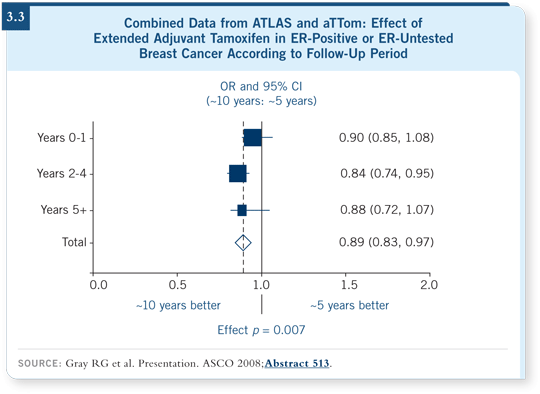
![]() DR ELLIS: With full acknowledgment that the effects seen are muted by the
inclusion of patients with hormone receptor-negative disease and that compliance
problems emerge with such long exposure to endocrine treatment, both
trials provide evidence that a longer duration of tamoxifen — essentially
beyond five years — is better than a shorter duration (Gray 2008; [3.3]).
DR ELLIS: With full acknowledgment that the effects seen are muted by the
inclusion of patients with hormone receptor-negative disease and that compliance
problems emerge with such long exposure to endocrine treatment, both
trials provide evidence that a longer duration of tamoxifen — essentially
beyond five years — is better than a shorter duration (Gray 2008; [3.3]).
![]() DR LOVE: How do you approach this decision clinically when you see a
patient who has completed five years of tamoxifen or an aromatase inhibitor?
DR LOVE: How do you approach this decision clinically when you see a
patient who has completed five years of tamoxifen or an aromatase inhibitor?
![]() DR ELLIS: I use a shamelessly risk-adapted approach. For patients at high risk,
I recommend 10 years of endocrine therapy.
DR ELLIS: I use a shamelessly risk-adapted approach. For patients at high risk,
I recommend 10 years of endocrine therapy.
You may ask, where’s the evidence for administering an aromatase inhibitor for 10 years? We don’t have any data yet. We have evidence for administering 10 years of tamoxifen. Certainly, continued letrozole appears more effective after five years of adjuvant tamoxifen (Goss 2005) than 10 years of tamoxifen, even in the most optimistic scenarios in aTTom and ATLAS.
Although we don’t have evidence to support administering 10 years of an aromatase inhibitor, they are more potent than tamoxifen and should, theoretically, provide even more benefit with longer durations.
For patients at lower risk, five years may be enough. Obviously, this becomes a long discussion with my patients after they’ve completed five years of endocrine therapy.
Track 15
![]() DR LOVE: Can you describe your study of estrogen at high and low doses
for metastatic breast cancer?
DR LOVE: Can you describe your study of estrogen at high and low doses
for metastatic breast cancer?
![]() DR ELLIS: We’ve recently completed a multicenter study with 66 patients,
evaluating 30 versus six milligrams of generic estradiol for patients who experienced disease progression while receiving an aromatase inhibitor. In this
study, if the patient benefits from estrogen therapy, she is switched back to the
aromatase inhibitor she was receiving before the progression.
DR ELLIS: We’ve recently completed a multicenter study with 66 patients,
evaluating 30 versus six milligrams of generic estradiol for patients who experienced disease progression while receiving an aromatase inhibitor. In this
study, if the patient benefits from estrogen therapy, she is switched back to the
aromatase inhibitor she was receiving before the progression.
The study is designed to determine whether oscillating between an aromatase inhibitor and estrogen therapy will produce a prolonged clinical benefit, and some tumors do appear to respond in that way. We will be presenting the data at the annual San Antonio Breast Cancer Symposium in December of this year.
Essentially, we’re seeing 10 to 15 percent actual responses — some quite dramatic — and approximately a 30 percent clinical benefit rate. Interestingly, the responses can be predicted by a PET flare.
We obtained a baseline PET image, and then 24 hours after treatment we examined the difference in glucose uptake. We found that the patients with a dramatic glucose uptake were the ones who went on to respond, so a biomarker for response does exist.
The 6-mg dose was as effective as and safer than the higher dose. We were careful to exclude patients with uncontrolled hypercalcemia and a history of thrombotic events or myocardial infarction.
In the trial, we saw no venous thrombotic events and found that the therapy was well tolerated. However, the flare reactions that you read about in textbooks are real.
Track 17
![]() DR LOVE: What was your take on the ASCO data from the AVADO trial
of docetaxel with or without bevacizumab as first-line therapy for patients
with locally recurrent or metastatic breast cancer (Miles 2008)?
DR LOVE: What was your take on the ASCO data from the AVADO trial
of docetaxel with or without bevacizumab as first-line therapy for patients
with locally recurrent or metastatic breast cancer (Miles 2008)?
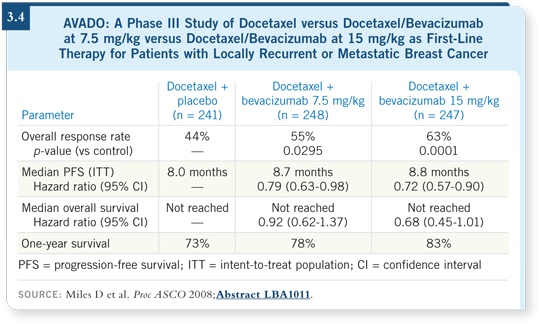
![]() DR ELLIS: I am concerned because progression-free survival is a difficult
and rather unstable endpoint, and in the ECOG-E2100 trial of paclitaxel and
bevacizumab, I see a disconnect between an amazing effect on progression-free
survival and almost no effect on overall survival (Miller 2007).
DR ELLIS: I am concerned because progression-free survival is a difficult
and rather unstable endpoint, and in the ECOG-E2100 trial of paclitaxel and
bevacizumab, I see a disconnect between an amazing effect on progression-free
survival and almost no effect on overall survival (Miller 2007).
The differences between the two arms in the AVADO trial seem narrow (Miles 2008; [3.4]) — not nearly as impressive as the ECOG-E2100 data (Miller 2007) — which underscores my point. I do believe that bevacizumab/taxane is an active combination, but I’m concerned about how much benefit you obtain versus the cost.
I reserve bevacizumab for patients in visceral crisis because those patients need a combination with a high response rate. A number of combinations can be used, such as paclitaxel with vinorelbine or gemcitabine, but I prefer paclitaxel with bevacizumab because I believe it has less toxicity and the E2100 data suggest that it’s somewhat better.
EDITOR'S NOTE
Austrian tag team
Neil Love, MD
- Select publications
INTERVIEWS
Michael Gnant, MD
- Select publications
Hyman B Muss, MD
- Select publications
Matthew J Ellis, MB, BChir, PhD
- Select publications
Charles L Vogel, MD (Fellows Rounds)
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity