
 |
||||||||

| Tracks 1-14 | ||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1-3
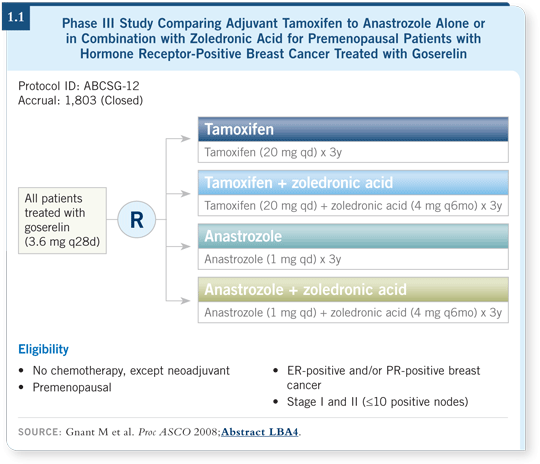
![]() DR LOVE: Can you discuss the Austrian Breast Cancer Study Group
(ABCSG) trial in premenopausal women you presented at ASCO this year?
DR LOVE: Can you discuss the Austrian Breast Cancer Study Group
(ABCSG) trial in premenopausal women you presented at ASCO this year?
![]() DR GNANT: The ABCSG-12 trial began with the issue of endocrine therapy.
However, we became concerned about what might happen to bone density
during aromatase inhibitor therapy. Our goal was to protect the bone, and we
thought bisphosphonates might also have antitumor activity, so we decided to
address both issues — endocrine therapy and the use of bisphosphonates — in
ABCSG-12 (Gnant 2008; [1.1]).
DR GNANT: The ABCSG-12 trial began with the issue of endocrine therapy.
However, we became concerned about what might happen to bone density
during aromatase inhibitor therapy. Our goal was to protect the bone, and we
thought bisphosphonates might also have antitumor activity, so we decided to
address both issues — endocrine therapy and the use of bisphosphonates — in
ABCSG-12 (Gnant 2008; [1.1]).
After our bone substudy was first reported in 2004 (Gnant 2004), we assembled an international advisory board to determine how to proceed. The bisphosphonates completely reversed bone loss from aromatase inhibitors, and among premenopausal patients, even tamoxifen could not completely protect the bone in those treated with goserelin.
Distinguished bone specialists such as Jean-Jacques Body and others said that we needed to answer the antitumor question. They were concerned that the sample size, which was 1,250 at the time, was not large enough. We decided to increase it to 1,800, knowing that we would have the power to detect at least a major effect from the bisphosphonate.
![]() DR LOVE: What were the eligibility requirements, and what kinds of patients
ended up enrolling?
DR LOVE: What were the eligibility requirements, and what kinds of patients
ended up enrolling?
![]() DR GNANT: Formally, the inclusion criteria were early-stage breast cancer and
endocrine-responsive disease, meaning ER-positive and/or PR-positive tumors.
We do not have a single patient in the trial with negative or unknown receptors.
The trial was open to patients with node-negative disease in addition to patients
with 10 or fewer positive nodes. In practice, physicians suggested participation in
this trial for patients with few affected nodes.
DR GNANT: Formally, the inclusion criteria were early-stage breast cancer and
endocrine-responsive disease, meaning ER-positive and/or PR-positive tumors.
We do not have a single patient in the trial with negative or unknown receptors.
The trial was open to patients with node-negative disease in addition to patients
with 10 or fewer positive nodes. In practice, physicians suggested participation in
this trial for patients with few affected nodes.
![]() DR LOVE: Can you discuss the results of the study?
DR LOVE: Can you discuss the results of the study?
![]() DR GNANT: At five years of follow-up, we observed only 137 disease-free
survival events. Obviously this good prognosis is wonderful for our patients,
but it’s not good for trialists. I believe it’s a mature trial. It has 1,800 patients,
with six to seven percent having experienced any event. I don’t believe that
these results will change in the future.
DR GNANT: At five years of follow-up, we observed only 137 disease-free
survival events. Obviously this good prognosis is wonderful for our patients,
but it’s not good for trialists. I believe it’s a mature trial. It has 1,800 patients,
with six to seven percent having experienced any event. I don’t believe that
these results will change in the future.
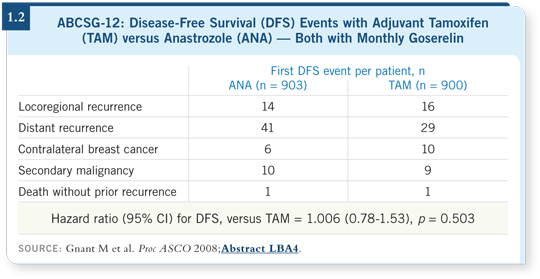
In her commentary at ASCO, Martine Piccart-Gebhart remarked that ABCSG-12 is underpowered to rule out the benefit of substituting anastrozole for tamoxifen. However, we showed that anastrozole and tamoxifen yielded strikingly similar results (Gnant 2008; [1.2]) in patients receiving an LHRH agonist.
![]() DR LOVE: One of the issues that Dr Piccart-Gebhart brought up was the
efficacy of ovarian suppression with an LHRH agonist. Did you evaluate that?
DR LOVE: One of the issues that Dr Piccart-Gebhart brought up was the
efficacy of ovarian suppression with an LHRH agonist. Did you evaluate that?
![]() DR GNANT: Only clinically. None of our patients was menstruating during
treatment. I agree with Martine that some patients may have more effective
estradiol suppression than others, but in terms of the clinical relevance
for daily practice, it’s well accepted that ovarian suppression works with the
monthly administration of goserelin.
DR GNANT: Only clinically. None of our patients was menstruating during
treatment. I agree with Martine that some patients may have more effective
estradiol suppression than others, but in terms of the clinical relevance
for daily practice, it’s well accepted that ovarian suppression works with the
monthly administration of goserelin.
![]() DR LOVE: Can you summarize what you saw in terms of the endocrine issue?
DR LOVE: Can you summarize what you saw in terms of the endocrine issue?
![]() DR GNANT: We observed no difference in disease-free survival, recurrence-free
survival or overall survival between anastrozole and tamoxifen in the
presence of ovarian suppression. The curves are virtually overlapping.
DR GNANT: We observed no difference in disease-free survival, recurrence-free
survival or overall survival between anastrozole and tamoxifen in the
presence of ovarian suppression. The curves are virtually overlapping.
Track 9
![]() DR LOVE: Can you discuss the dosing schedule and results of zoledronic
acid in ABCSG-12?
DR LOVE: Can you discuss the dosing schedule and results of zoledronic
acid in ABCSG-12?
![]() DR GNANT: We administered four milligrams of zoledronic acid every six
months, for a total of seven infusions over three years. Initially, we started the trial with a higher dose of eight milligrams monthly, but we were forced to
change due to safety concerns. In 2000, reports surfaced that renal safety was
endangered in some patients with multiple myeloma who were being treated
with zoledronic acid, and at that point all the trials around the world reduced
the dose to four milligrams.
DR GNANT: We administered four milligrams of zoledronic acid every six
months, for a total of seven infusions over three years. Initially, we started the trial with a higher dose of eight milligrams monthly, but we were forced to
change due to safety concerns. In 2000, reports surfaced that renal safety was
endangered in some patients with multiple myeloma who were being treated
with zoledronic acid, and at that point all the trials around the world reduced
the dose to four milligrams.
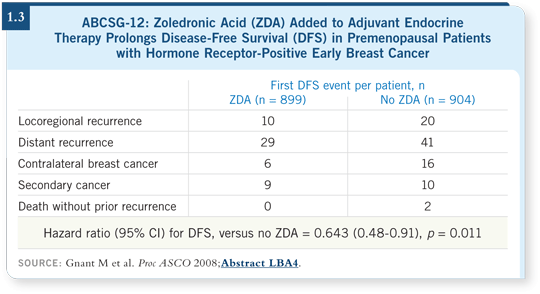
We went back to what we believed would be mostly a bone-protection dose. Therefore, it’s particularly striking that we are not only protecting bone at this dose but that we are also keeping the cancer at bay (Gnant 2008; [1.3]).
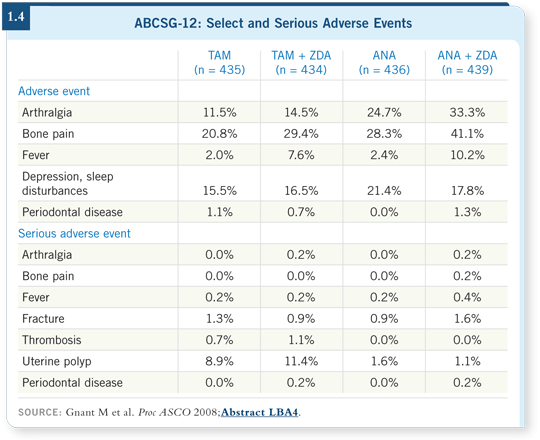
Two more observations are also exciting. One is the magnitude of the effect: A 36 percent improvement in disease-free survival, translating to at least a non-significant trend toward better overall survival. That’s an accomplishment usually observed with interventions such as taxane chemotherapy. We observed that efficacy with an acceptable side-effect profile (1.4).
More importantly, we’re not only preventing bone metastases, but we’re also seeing benefit in various event subcategories, including locoregional recurrence, contralateral breast cancer and distant metastasis outside of the bone (such as liver or lung disease). That’s something most of us did not expect.
Track 11
![]() DR LOVE: What about osteonecrosis of the jaw (ONJ)?
DR LOVE: What about osteonecrosis of the jaw (ONJ)?
![]() DR GNANT: When we started the trial in 1999, nobody was aware of ONJ.
When the first reports were published, we made an effort to educate physicians
and patients, saying, “Be careful with your dental procedures. Report
symptoms early.”
DR GNANT: When we started the trial in 1999, nobody was aware of ONJ.
When the first reports were published, we made an effort to educate physicians
and patients, saying, “Be careful with your dental procedures. Report
symptoms early.”
We identified three suspected cases and examined the original dental films. We did not find evidence of a single case of confirmed ONJ (1.4). This is in line with what is known about that dose and frequency of administration of zoledronic acid. Basically, all the reports suggest that ONJ with IV bisphosphonates occurs with more intense regimens or higher-dose schedules. I would say that ONJ is not a problem in the adjuvant treatment setting.
![]() DR LOVE: Robert Marx, who was one of the oral surgeons involved in identifying
ONJ, makes the recommendation that patients who receive bisphosphonates
should see a dentist prior to initiating the bisphosphonate to ensure that
they don’t have any major problems (Marx 2007). Is that your approach?
DR LOVE: Robert Marx, who was one of the oral surgeons involved in identifying
ONJ, makes the recommendation that patients who receive bisphosphonates
should see a dentist prior to initiating the bisphosphonate to ensure that
they don’t have any major problems (Marx 2007). Is that your approach?
![]() DR GNANT: Yes, I support that recommendation. I believe that it’s prudent to
recommend a dental exam for everyone ahead of receiving bisphosphonates.
DR GNANT: Yes, I support that recommendation. I believe that it’s prudent to
recommend a dental exam for everyone ahead of receiving bisphosphonates.
Track 12
![]() DR LOVE: Are the results of your study ready for prime time? Martine
was somewhat cautious (1.5), suggesting that we need to wait for another
study before considering ZDA off protocol.
DR LOVE: Are the results of your study ready for prime time? Martine
was somewhat cautious (1.5), suggesting that we need to wait for another
study before considering ZDA off protocol.
![]() DR GNANT: I see two sides to that answer. As a scientist, I would like to have
confirmation for everything I do. At least for now, the application of these
results should be confined to the population in which they were derived. We
should be careful in extrapolating them to other patient populations, such as
patients with hormone receptor-negative disease.
DR GNANT: I see two sides to that answer. As a scientist, I would like to have
confirmation for everything I do. At least for now, the application of these
results should be confined to the population in which they were derived. We
should be careful in extrapolating them to other patient populations, such as
patients with hormone receptor-negative disease.
![]() DR LOVE: “Careful” as in don’t do it?
DR LOVE: “Careful” as in don’t do it?
![]() DR GNANT: Probably for now, particularly because we will have confirmatory
data soon. The AZURE (BIG 1-04) study, which is examining zoledronic
acid in pre-and postmenopausal women with node-positive disease, is
expected to report by the end of this year in San Antonio.
DR GNANT: Probably for now, particularly because we will have confirmatory
data soon. The AZURE (BIG 1-04) study, which is examining zoledronic
acid in pre-and postmenopausal women with node-positive disease, is
expected to report by the end of this year in San Antonio.
![]() DR LOVE: In hormone receptor-negative and hormone receptor-positive
disease?
DR LOVE: In hormone receptor-negative and hormone receptor-positive
disease?
![]() DR GNANT: Yes, in both, with at least 20 percent of patients having hormone
receptor-negative disease. Robert E Coleman is the principal investigator.
DR GNANT: Yes, in both, with at least 20 percent of patients having hormone
receptor-negative disease. Robert E Coleman is the principal investigator.
They have recruited 3,300 patients, and they will conduct the first analysis in September. So that’s the scientific interpretation: It’s prudent to be conservative.
On the other hand, I will answer as a doctor and as a person. Obviously we don’t have approval for any of these practices currently, so patients may come across availability and reimbursement issues.
But frankly, if my sister were diagnosed next week — if she were premenopausal and had endocrine-responsive disease — she would evaluate the data and say, “That’s seven infusions of 50 minutes each over the course of three years.

It’s well tolerated. The toxicity is either low or nonexistent, and what to watch out for is well defined. It’s already been proved effective in protecting bone against the side effects of endocrine treatment, and it’s now been shown to keep the cancer at bay by an additional one third. Let me have that.” It would be a struggle for me to say, “Wait for approval. Wait for another 12 months.”
![]() DR LOVE: What if it were your older, postmenopausal sister?
DR LOVE: What if it were your older, postmenopausal sister?
![]() DR GNANT: That’s a little more difficult. I would try to stay disciplined and
say that we will know the answer in six months. We can probably start the
bisphosphonate in six months, once we demonstrate the positive effects, so let’s
wait.
DR GNANT: That’s a little more difficult. I would try to stay disciplined and
say that we will know the answer in six months. We can probably start the
bisphosphonate in six months, once we demonstrate the positive effects, so let’s
wait.
EDITOR'S NOTE
Austrian tag team
Neil Love, MD
- Select publications
INTERVIEWS
Michael Gnant, MD
- Select publications
Hyman B Muss, MD
- Select publications
Matthew J Ellis, MB, BChir, PhD
- Select publications
Charles L Vogel, MD (Fellows Rounds)
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity