
 |
||||||||

| Tracks 1-14 | ||||||||||||||||||||||||||||||
|
(See Audio Program for Interviews with These Patients)

Tracks 1-3
![]() DR VOGEL: This patient was diagnosed with hormone receptor-positive,
HER2-positive early breast cancer in the early 1990s, for which she underwent
bilateral mastectomies followed by adjuvant chemotherapy.
DR VOGEL: This patient was diagnosed with hormone receptor-positive,
HER2-positive early breast cancer in the early 1990s, for which she underwent
bilateral mastectomies followed by adjuvant chemotherapy.
Approximately one year later, she developed extensive bony metastases and was enrolled on the pivotal trastuzumab trial (Slamon 2001).
After her disease progressed, she was enrolled on the extension trial and received cisplatin/trastuzumab. Subsequently she was treated with vinorelbine/ trastuzumab, but her longest response to therapy was seven years while receiving toremifene and trastuzumab after she underwent an oophorectomy. She stayed in remission from 1998 to 2005.
![]() DR LOVE: Obviously this is an unusual case. She has been treated for
metastatic breast cancer for the past 13 years entirely on an outpatient basis,
and she appears to be completely healthy. What has happened to her recently?
DR LOVE: Obviously this is an unusual case. She has been treated for
metastatic breast cancer for the past 13 years entirely on an outpatient basis,
and she appears to be completely healthy. What has happened to her recently?
![]() DR VOGEL: We decided to keep her on hormonal therapy for as long as
possible, so after her disease progressed on toremifene she was treated with
exemestane, then fulvestrant — all in combination with continued trastuzumab.
DR VOGEL: We decided to keep her on hormonal therapy for as long as
possible, so after her disease progressed on toremifene she was treated with
exemestane, then fulvestrant — all in combination with continued trastuzumab.
Most recently, in May 2007, we elected to continue trastuzumab and treat her with capecitabine/lapatinib. Her bone lesions have been stable for the past year, but she has suffered from a troublesome acneiform rash.
![]() DR LOVE: What side effects have you observed with lapatinib?
DR LOVE: What side effects have you observed with lapatinib?
![]() DR VOGEL: We have seen some liver function test abnormalities, which is
apparently a new finding, but they resolve when the drug is discontinued.
Except for the current patient, rash is not usually a problem. Diarrhea has been
a troubling complication for some of my patients, but we have not observed
other significant issues.
DR VOGEL: We have seen some liver function test abnormalities, which is
apparently a new finding, but they resolve when the drug is discontinued.
Except for the current patient, rash is not usually a problem. Diarrhea has been
a troubling complication for some of my patients, but we have not observed
other significant issues.
Track 4
![]() DR LOVE: This patient is an extreme example of continuing trastuzumab
in combination with other therapies for the treatment of HER2-positive
metastatic breast cancer. Do you have other patients like her, for whom
trastuzumab was continued through multiple lines of therapy (4.1)?
DR LOVE: This patient is an extreme example of continuing trastuzumab
in combination with other therapies for the treatment of HER2-positive
metastatic breast cancer. Do you have other patients like her, for whom
trastuzumab was continued through multiple lines of therapy (4.1)?
![]() DR VOGEL: I have several such patients. One patient with bone metastases
has been treated for 10 years, and she refuses to come off treatment. Another
patient has been treated with liver metastases for eight years.
DR VOGEL: I have several such patients. One patient with bone metastases
has been treated for 10 years, and she refuses to come off treatment. Another
patient has been treated with liver metastases for eight years.
![]() DR LOVE: Do you have any patients who have experienced relapse after treatment
with adjuvant trastuzumab? How do you determine whether to restart
trastuzumab, use lapatinib or both?
DR LOVE: Do you have any patients who have experienced relapse after treatment
with adjuvant trastuzumab? How do you determine whether to restart
trastuzumab, use lapatinib or both?
![]() DR VOGEL: I have four such patients. I govern my approach by the time to relapse after cessation of trastuzumab. If the relapse occurs within one year,
then I treat with lapatinib.
DR VOGEL: I have four such patients. I govern my approach by the time to relapse after cessation of trastuzumab. If the relapse occurs within one year,
then I treat with lapatinib.

Tracks 5, 7-9
![]() DR VOGEL: This woman was 40 years old when she was diagnosed with
breast cancer in 1996. She underwent a modified mastectomy and had 25
out of 36 positive nodes. She received adjuvant AC followed by docetaxel,
comprehensive nodal and chest wall irradiation therapy and oophorectomy
with tamoxifen, which she discontinued after three months solely due to hot
flashes.
DR VOGEL: This woman was 40 years old when she was diagnosed with
breast cancer in 1996. She underwent a modified mastectomy and had 25
out of 36 positive nodes. She received adjuvant AC followed by docetaxel,
comprehensive nodal and chest wall irradiation therapy and oophorectomy
with tamoxifen, which she discontinued after three months solely due to hot
flashes.
In 2004 she developed a painful recurrence in her bone and underwent hemipelvic radiation therapy. She was enrolled on a clinical trial with letrozole and also received the Theratope® vaccine.
Unfortunately, her disease progressed quickly, and she was enrolled on EFECT, on which she received exemestane. After seven months her disease progressed again and was treated with fulvestrant, but she developed liver metastases after two months.
![]() DR LOVE: At the point when you felt this woman would not respond to
hormonal therapy, you approached her about enrollment on the RIBBON 1 trial (4.2)?
DR LOVE: At the point when you felt this woman would not respond to
hormonal therapy, you approached her about enrollment on the RIBBON 1 trial (4.2)?
![]() DR VOGEL: Yes. She was still experiencing pain while on fulvestrant, and
during a workup we were surprised to find liver metastases, so we felt it was
probably better to treat her with chemotherapy.
DR VOGEL: Yes. She was still experiencing pain while on fulvestrant, and
during a workup we were surprised to find liver metastases, so we felt it was
probably better to treat her with chemotherapy.
I chose capecitabine for nearly all of my patients enrolled on RIBBON 1. It meshes with my basic philosophy of care to use the least-toxic chemotherapy possible. She received capecitabine with either bevacizumab or placebo. She’s had the longest response of my patients on RIBBON 1 receiving capecitabine, and she continues on the regimen now approaching two years.
Patients enrolled on RIBBON 1, after their disease progressed on initial therapy such as capecitabine with or without bevacizumab, had the option after that of receiving open-label bevacizumab with chemotherapy. I chose nab paclitaxel as my drug of choice for the postprogression phase of the study. Most of these patients have responded to nab paclitaxel with open-label bevacizumab and have fared nicely on that regimen.
I’m more impressed with that regimen than I am with capecitabine/bevacizumab. I’m aware of the XCaliBr data, in which patients with ER-positive disease seemed to fare better on capecitabine and bevacizumab than those with ER-negative disease (Sledge 2007).
![]() DR LOVE: What is your opinion about nab paclitaxel with bevacizumab
compared to paclitaxel with bevacizumab?
DR LOVE: What is your opinion about nab paclitaxel with bevacizumab
compared to paclitaxel with bevacizumab?
![]() DR VOGEL: Wherever possible, I use nab paclitaxel rather than paclitaxel.
DR VOGEL: Wherever possible, I use nab paclitaxel rather than paclitaxel.
I believe it’s possible that nab paclitaxel may represent a superior way of administering paclitaxel, and you avoid the longer infusions associated with paclitaxel. Because of all the premedications required for paclitaxel, I believe nab paclitaxel is probably better tolerated.
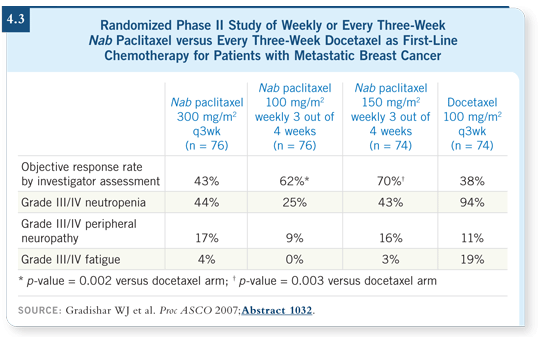
![]() DR LOVE: What do you think about the Phase II randomized trial results
presented by Dr Gradishar, suggesting that nab paclitaxel was more effective
than docetaxel (Gradishar 2007; [4.3])?
DR LOVE: What do you think about the Phase II randomized trial results
presented by Dr Gradishar, suggesting that nab paclitaxel was more effective
than docetaxel (Gradishar 2007; [4.3])?
![]() DR VOGEL: That trial is being repeated here in the United States. It would
certainly be interesting if it showed the same results. It is a Phase III randomized
trial, as I understand it, and a trial that is truly needed. The science is
compelling, and the preliminary data are interesting. I wouldn’t be surprised
if nab paclitaxel proves superior to paclitaxel, and I wouldn’t be surprised if it
were superior to docetaxel, either.
DR VOGEL: That trial is being repeated here in the United States. It would
certainly be interesting if it showed the same results. It is a Phase III randomized
trial, as I understand it, and a trial that is truly needed. The science is
compelling, and the preliminary data are interesting. I wouldn’t be surprised
if nab paclitaxel proves superior to paclitaxel, and I wouldn’t be surprised if it
were superior to docetaxel, either.

Track 10
![]() DR VOGEL: This patient had advanced disease, with bone, liver and nodal
metastases. She was treated with paclitaxel/bevacizumab and went into a nice
remission.
DR VOGEL: This patient had advanced disease, with bone, liver and nodal
metastases. She was treated with paclitaxel/bevacizumab and went into a nice
remission.
She developed fatigue, neuropathy and weight gain. She gained 35 pounds after starting therapy.
I believed the weight gain was probably a side effect of the steroid administered with paclitaxel. We decided she needed a break, so we put her back on hormonal therapy but were unable to maintain the remission, although she lost the weight she had gained previously.
When we decided to reinitiate chemotherapy and bevacizumab, I administered nab paclitaxel, which she has tolerated much better (4.4) and without weight gain.

Tracks 12-14
![]() DR VOGEL: This patient is a quiet, soft-spoken nurse who was diagnosed
with de novo Stage IV disease in 2003, and she received sequential hormonal
therapy for several years with letrozole/goserelin, fulvestrant/goserelin and
exemestane/goserelin.
DR VOGEL: This patient is a quiet, soft-spoken nurse who was diagnosed
with de novo Stage IV disease in 2003, and she received sequential hormonal
therapy for several years with letrozole/goserelin, fulvestrant/goserelin and
exemestane/goserelin.
She developed liver and bone metastases and was treated on a clinical trial evaluating docetaxel with capecitabine. Her disease showed a good response, but she experienced toxicity from the combination.
Then she received tamoxifen with goserelin for a year and a half. When her disease progressed in May 2007, she received high-dose estrogen with goserelin, but unfortunately she did not respond.
So we were completing a workup to start her on the RIBBON 2 trial, which is chemotherapy of a number of different types with or without bevacizumab in the second-line chemotherapy setting. If I intend to treat a patient with bevacizumab, it’s my policy to ensure that brain metastases are not present. Lo and behold, she had relatively small, asymptomatic brain lesions, so she was ineligible for that trial.
Her tumor did not progress on docetaxel/capecitabine, so we decided to treat with capecitabine. The question was, what do we do with the asymptomatic brain metastases?
I toyed with the idea of treating her with the gamma knife but decided that because these lesions were not bothering her, we would simply observe her on capecitabine alone and would not treat her with radiation therapy of any sort.
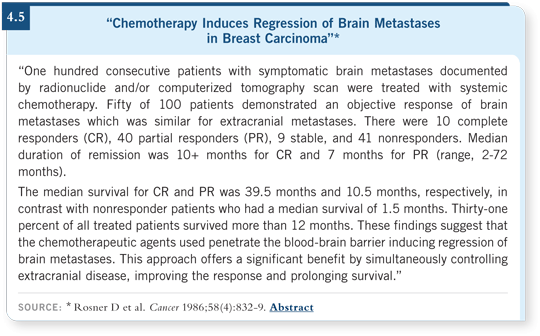
The precedent for this approach came from an old study by Dutzu Rosner in the 1980s, in which he used CMF/VP for patients with brain metastases who had not undergone radiation therapy (Rosner 1983, 1986; [4.5]). He demonstrated a definite response rate, so it appears that the blood-brain barrier may not necessarily be intact in patients with brain metastases.
So we treated her with capecitabine and monitored her brain closely. Her first MRI of the brain revealed disappearance of one of the nodules and stability of another.
She continues to be asymptomatic, her liver lesions are improving and her tumor markers are declining on single-agent capecitabine for bone, liver and brain metastases. She has no side effects and is responding beautifully.
EDITOR'S NOTE
Austrian tag team
Neil Love, MD
- Select publications
INTERVIEWS
Michael Gnant, MD
- Select publications
Hyman B Muss, MD
- Select publications
Matthew J Ellis, MB, BChir, PhD
- Select publications
Charles L Vogel, MD (Fellows Rounds)
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity