
 |
||||||||

| Tracks 1-17 | ||||||||||||||||||||||||||||||||||||
|
Case Discussion with Drs Vogel and Muss

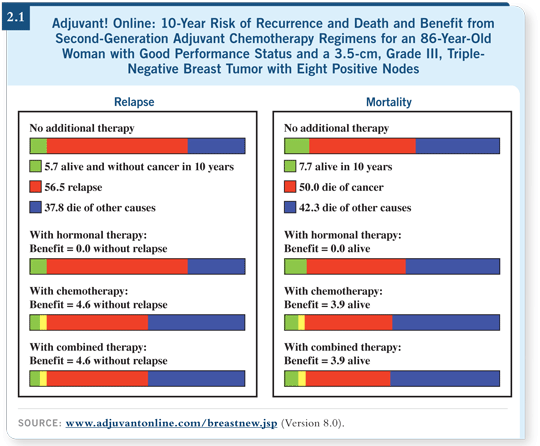
![]() DR LOVE: Dr Adler showed this patient the Adjuvant! Online data, which
suggest a modest benefit in mortality and recurrence over 10 years with
“second-generation” adjuvant chemotherapy (2.1). She understands that this is
ER-negative/PR-negative disease and that if it recurs, it may be in the next
two to three years.
DR LOVE: Dr Adler showed this patient the Adjuvant! Online data, which
suggest a modest benefit in mortality and recurrence over 10 years with
“second-generation” adjuvant chemotherapy (2.1). She understands that this is
ER-negative/PR-negative disease and that if it recurs, it may be in the next
two to three years.
Dr Adler’s question is, “What are your thoughts about the benefits of therapy for this elderly woman, and if you would treat her, which regimen would you utilize?”
![]() DR VOGEL: I haven’t treated many 86-year-olds with chemotherapy. This lady
has a poor prognosis across the board. On the other hand, her likelihood of
dying of other causes according to Adjuvant! Online is extraordinarily high.
DR VOGEL: I haven’t treated many 86-year-olds with chemotherapy. This lady
has a poor prognosis across the board. On the other hand, her likelihood of
dying of other causes according to Adjuvant! Online is extraordinarily high.
It’s a tough case because this woman is different from the average 86-year-old in that she is physically well and strongly desires chemotherapy. Most elderly women wouldn’t even consider chemotherapy. I would probably suggest observation, but if she persisted in wanting to be aggressive, then I would probably treat her.
I don’t believe I would use anything beyond docetaxel/cyclophosphamide (TC) for four cycles with pegfilgrastim, which circumvents the doxorubicin-associated concerns about cardiac dysfunction and leukemia in addition to emerging issues related to the value of doxorubicin for patients whose disease is almost certainly TOPO II-negative.
I haven’t treated many patients over eighty with adjuvant TC, but I have treated patients in their seventies. In accord with the guidelines, we add pegfilgrastim for patients older than age 65, even though the rate of febrile neutropenia in Steve Jones’s study was much below that which would mandate prophylactic growth factor support.
![]() DR MUSS: An 86-year-old woman in perfect health might have a five-year
average survival, and her risk of recurrence with this disease is probably
substantial within that five-year range. Approximately 70 to 80 percent of the
recurrences will occur in that five-year range.
DR MUSS: An 86-year-old woman in perfect health might have a five-year
average survival, and her risk of recurrence with this disease is probably
substantial within that five-year range. Approximately 70 to 80 percent of the
recurrences will occur in that five-year range.
If she received TC chemotherapy, she may have a one third proportional reduction in risk. I estimate that she might increase her chance of survival by a few percent. If she were interested in that benefit, then I might consider something like adjuvant TC and pegfilgrastim.
However, it’s “iffy.” It would interfere with her quality of life, and she would experience some toxicity. She would need to be in perfect health, and I would let her know about my ambivalence in treating her.
Select Excerpts from the Interview with Dr Muss
Track 4
![]() DR LOVE: Can you discuss the analysis you did of the effect of TC in
older versus younger patients?
DR LOVE: Can you discuss the analysis you did of the effect of TC in
older versus younger patients?
![]() DR MUSS: I’m not a US Oncology member, but I met with the principal
investigator of the 9735 trial and said, “Steve, you have a huge study here. You
have a number of elderly patients on this study. No one has data on this. Let’s
evaluate it.” We ended up studying those 167 patients.
DR MUSS: I’m not a US Oncology member, but I met with the principal
investigator of the 9735 trial and said, “Steve, you have a huge study here. You
have a number of elderly patients on this study. No one has data on this. Let’s
evaluate it.” We ended up studying those 167 patients.
I’m meticulous, so we conducted a multivariate analysis. I wanted to make sure that it was not a quirk, and sure enough, those older patients experienced a survival advantage. The paper was sent recently to the Journal of Clinical Oncology.
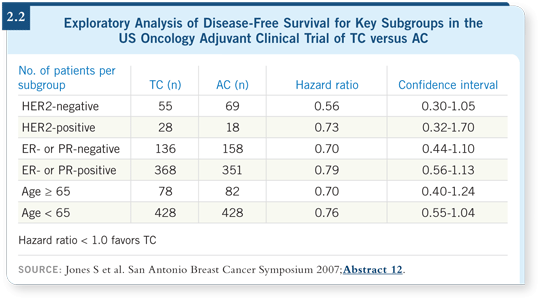
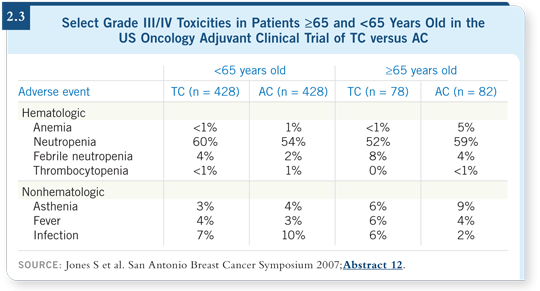
The older patients exhibited similar proportional benefits to the younger patients. They have poorer overall survival because of competing causes of death, but their proportional benefit in relapse is similar to that of younger patients (2.2). They experienced a little more toxicity — eight percent versus four percent neutropenic fever (2.3). I tend to use growth factors from the beginning with these patients. I’m using more TC in general in my practice.
![]() DR LOVE: What age would cause you to start using growth factors with TC?
DR LOVE: What age would cause you to start using growth factors with TC?
![]() DR MUSS: I believe age 65 is a reasonable cutoff, possibly younger for a
patient with substantial comorbidities — for example, patients with COPD
who may develop pneumonia and become septic.
DR MUSS: I believe age 65 is a reasonable cutoff, possibly younger for a
patient with substantial comorbidities — for example, patients with COPD
who may develop pneumonia and become septic.
![]() DR LOVE: How do patients in their seventies tolerate TC in your practice?
DR LOVE: How do patients in their seventies tolerate TC in your practice?
![]() DR MUSS: From our data, TC is well tolerated. We see fatigue with all
chemotherapy, but I believe more fatigue is associated with docetaxel,
whatever the mechanism — whether it’s making more IL6 or TNF, or
whatever is happening — it’s a bit tougher on patients.
DR MUSS: From our data, TC is well tolerated. We see fatigue with all
chemotherapy, but I believe more fatigue is associated with docetaxel,
whatever the mechanism — whether it’s making more IL6 or TNF, or
whatever is happening — it’s a bit tougher on patients.
The nice facet is that you’re not worried about a cardiac toxicity profile. The neutropenia is of concern when you use 75 mg/m2 of docetaxel: Most patients become neutropenic, but neutropenic fever has been uncommon.
Track 5
![]() DR LOVE: What do we know about the relationship between chemotherapy-induced leukemia and age?
DR LOVE: What do we know about the relationship between chemotherapy-induced leukemia and age?
![]() DR MUSS: If you evaluate the SEER data on women older than age 65, you
find that about one percent have a risk of AML. The hazard ratio if you focus
on chemotherapy is in the 1.6 to 2.0 range, and with anthracyclines the hazard
ratio is approximately 2.1 to 2.2. So I believe concern does arise with age and
anthracyclines, which are associated with those chromosomal abnormalities for
TOPO II-inhibiting agents and leukemia. Those leukemias tend to occur four
to eight years out.
DR MUSS: If you evaluate the SEER data on women older than age 65, you
find that about one percent have a risk of AML. The hazard ratio if you focus
on chemotherapy is in the 1.6 to 2.0 range, and with anthracyclines the hazard
ratio is approximately 2.1 to 2.2. So I believe concern does arise with age and
anthracyclines, which are associated with those chromosomal abnormalities for
TOPO II-inhibiting agents and leukemia. Those leukemias tend to occur four
to eight years out.
When we evaluated our CALGB data for toxicity and treatment-related deaths among the elderly, we found MDS and AML to be a major problem. The issues were not heart related, probably because we put healthy patients on the trial. The incidence of AML or MDS was 0.7 percent in the most recent update of our CALGB-9741 trial (Muss 2007), which is high. When you enter all of these factors into Adjuvant! Online and a one percent survival benefit appears, administering chemotherapy is something you really have to consider.
Track 8
![]() DR LOVE: Would you discuss the CALGB-49907 randomized Phase III
study focusing on the elderly that you reported at ASCO 2008 (Muss
2008)?
DR LOVE: Would you discuss the CALGB-49907 randomized Phase III
study focusing on the elderly that you reported at ASCO 2008 (Muss
2008)?
![]() DR MUSS: We compared standard chemotherapy — physicians could choose
CMF with oral cyclophosphamide or AC as defined by the NSABP — to
capecitabine administered orally for two consecutive weeks out of every three
weeks. To be eligible, patients of any nodal and HER2 status had to be 65
years of age or older, have a tumor greater than or equal to one centimeter, an
estimated survival of five years and normal organ function.
DR MUSS: We compared standard chemotherapy — physicians could choose
CMF with oral cyclophosphamide or AC as defined by the NSABP — to
capecitabine administered orally for two consecutive weeks out of every three
weeks. To be eligible, patients of any nodal and HER2 status had to be 65
years of age or older, have a tumor greater than or equal to one centimeter, an
estimated survival of five years and normal organ function.
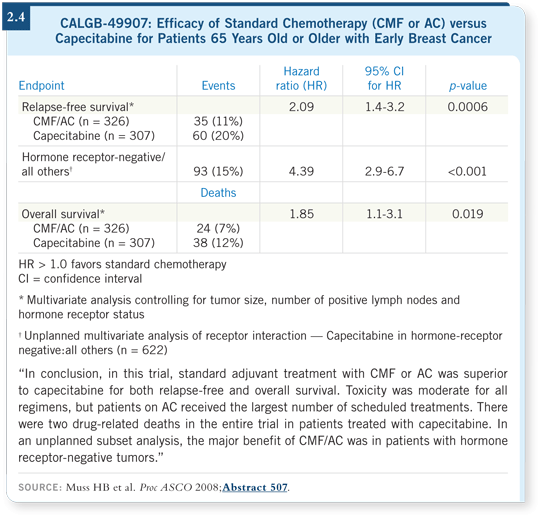
The trial was performed based on data from trials in metastatic breast cancer under the hypothesis that single-agent capecitabine would be noninferior. This was a clever, adaptive and unique design by Don Berry based on Bayesian statistics. We selected a specific point, between 600 patients and a maximum of 1,800 patients, to calculate what the likelihood would be that capecitabine was noninferior to AC.
We also had cutoffs at which we would stop the trial if the capecitabine was inferior or likely to be inferior. In November of 2006, after accruing 600 patients, we performed our first analysis and found it likely that capecitabine was inferior to CMF or AC, and we halted accrual. Analysis of the data revealed a highly significant benefit in both relapse-free and overall survival for patients treated with standard chemotherapy, either classic CMF or AC, compared to patients treated with capecitabine (2.4).
We also performed an unplanned subset analysis and found an interaction between treatment and outcome. Specifically, patients with hormone receptor-negative — ER-negative, PR-negative — disease obtained the greatest value from CMF or AC treatment compared to capecitabine. For patients with hormone receptor-positive disease, the difference was not as obvious.
Track 9
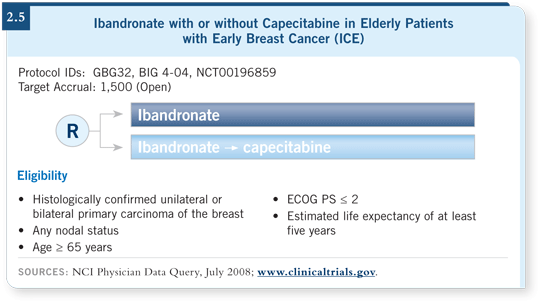
![]() DR LOVE: Would you discuss the ICE trial, which is evaluating ibandronate
with or without capecitabine?
DR LOVE: Would you discuss the ICE trial, which is evaluating ibandronate
with or without capecitabine?
![]() DR MUSS: This is an ongoing European trial led by the German group, in
which patients with hormone receptor-positive and hormone receptor-negative
disease receive ibandronate and are then randomly assigned to capecitabine or
no additional treatment (2.5). I met Dr Von Minckwitz, chair of the German
group, at the ASCO meeting, and he told me that they have accrued approximately
1,350 of the planned 1,500 patients. This trial will tell us a lot about the
use of the oral agent and will provide important data in the context of our trial.
DR MUSS: This is an ongoing European trial led by the German group, in
which patients with hormone receptor-positive and hormone receptor-negative
disease receive ibandronate and are then randomly assigned to capecitabine or
no additional treatment (2.5). I met Dr Von Minckwitz, chair of the German
group, at the ASCO meeting, and he told me that they have accrued approximately
1,350 of the planned 1,500 patients. This trial will tell us a lot about the
use of the oral agent and will provide important data in the context of our trial.
Their patient population may be at lower risk than ours, but I believe that early on, many of the patients will fare well and will probably gain little benefit from the chemotherapy. The patients with hormone receptor-positive disease receive endocrine therapy. For the patients with hormone receptor-negative disease, I’d like to believe that capecitabine will have value. It may not be as great a value as more aggressive chemotherapy, however.
Evidence supporting that theory comes from Don Berry’s analysis in JAMA evaluating patients with ER-negative, PR-negative disease treated on the CALGB trials. He reported that as you use more aggressive chemotherapy and taxanes and progress to dose-dense therapy, you observe more improved proportional reductions in the relapse rate among patients with ER-negative, PR-negative disease (Berry 2006).
So I’d like to believe that capecitabine would be helpful, but it wouldn’t be as effective as a more modern, more aggressive regimen.
Tracks 10-11
![]() DR LOVE: What is your treatment algorithm for the management of
metastatic disease in the older patient, and does it change when the patient
reaches age 70 or 80?
DR LOVE: What is your treatment algorithm for the management of
metastatic disease in the older patient, and does it change when the patient
reaches age 70 or 80?
![]() DR MUSS: I don’t think so. I use a lot of capecitabine in the first-line setting.
Capecitabine is an excellent choice to initiate chemotherapy in metastatic
breast cancer, irrespective of age. It’s well tolerated, though I start with a lower
dose. I don’t believe enough data exist to tell me the ideal dose to use. The
package insert dose is too high.
DR MUSS: I don’t think so. I use a lot of capecitabine in the first-line setting.
Capecitabine is an excellent choice to initiate chemotherapy in metastatic
breast cancer, irrespective of age. It’s well tolerated, though I start with a lower
dose. I don’t believe enough data exist to tell me the ideal dose to use. The
package insert dose is too high.
Many of my patients say, “Dr Muss, I don’t want any chemotherapy and I certainly don’t want to get sick.” I try to reassure them that an oral agent is available, that it’s real chemotherapy, and I start it at a low dose. I can always escalate it later, but if the patient develops a terrible toxicity from the beginning, I’ve lost a good option.
![]() DR LOVE: What about the issue of capecitabine versus a taxane alone or with
bevacizumab in the first-line metastatic setting?
DR LOVE: What about the issue of capecitabine versus a taxane alone or with
bevacizumab in the first-line metastatic setting?
![]() DR MUSS: In Kathy Miller’s pivotal ECOG-E2100 trial evaluating paclitaxel with or without bevacizumab, a meaningful improvement was observed in
progression-free survival (Miller 2007). Without an overall survival benefit
in a clean trial, this tells me that I can use kinder, gentler therapy with
capecitabine up front without compromising the longevity of my patient.
DR MUSS: In Kathy Miller’s pivotal ECOG-E2100 trial evaluating paclitaxel with or without bevacizumab, a meaningful improvement was observed in
progression-free survival (Miller 2007). Without an overall survival benefit
in a clean trial, this tells me that I can use kinder, gentler therapy with
capecitabine up front without compromising the longevity of my patient.
I lean toward paclitaxel/bevacizumab for a patient with more extensive metastases, but I suspect that most of the patients we see today are minimally symptomatic, especially with physicians who still prefer numerous scans and follow-up studies. In that situation, you don’t have to be highly aggressive, worrying about a tumor doubling and impairing your opportunity to treat with capecitabine.
![]() DR LOVE: So for a patient with metastatic disease who has never received a
taxane, you would administer capecitabine? At some point, if she experiences
disease progression, will you then administer a taxane and bevacizumab?
DR LOVE: So for a patient with metastatic disease who has never received a
taxane, you would administer capecitabine? At some point, if she experiences
disease progression, will you then administer a taxane and bevacizumab?
![]() DR MUSS: Yes, and I realize that the data don’t exist to support me in the
second-line setting, but I have done that.
DR MUSS: Yes, and I realize that the data don’t exist to support me in the
second-line setting, but I have done that.
![]() DR LOVE: What are your thoughts on combining nab paclitaxel with
bevacizumab?
DR LOVE: What are your thoughts on combining nab paclitaxel with
bevacizumab?
![]() DR MUSS: I believe that nab paclitaxel is an exciting drug. It has a different
mechanism of action, but it’s never been compared to weekly paclitaxel, only
to docetaxel in Bill Gradishar’s randomized Phase II trials (Gradishar 2007).
I use paclitaxel, but if we see allergic reactions or other issues, I switch to nab
paclitaxel.
DR MUSS: I believe that nab paclitaxel is an exciting drug. It has a different
mechanism of action, but it’s never been compared to weekly paclitaxel, only
to docetaxel in Bill Gradishar’s randomized Phase II trials (Gradishar 2007).
I use paclitaxel, but if we see allergic reactions or other issues, I switch to nab
paclitaxel.
I would love to see a randomized trial evaluating nab paclitaxel. The CALGB is launching a study in the metastatic setting in which all patients will receive bevacizumab and will then be randomly assigned to receive nab paclitaxel, paclitaxel or ixabepilone. We will learn a lot from that trial.
Track 15
![]() DR LOVE: One of the adjuvant issues that’s been debated with older
patients or those with comorbidities is using trastuzumab alone, particularly
for patients with comorbidities for whom you normally wouldn’t
consider chemotherapy. What do you think about that strategy?
DR LOVE: One of the adjuvant issues that’s been debated with older
patients or those with comorbidities is using trastuzumab alone, particularly
for patients with comorbidities for whom you normally wouldn’t
consider chemotherapy. What do you think about that strategy?
![]() DR MUSS: It would be great to have a clinical trial of trastuzumab versus not,
especially for older patients, although I believe it would be hard to complete
conceptually because if patients were healthy, they would receive chemotherapy
and trastuzumab.
DR MUSS: It would be great to have a clinical trial of trastuzumab versus not,
especially for older patients, although I believe it would be hard to complete
conceptually because if patients were healthy, they would receive chemotherapy
and trastuzumab.
You’d be left with patients who were sicker and more frail, and then you’d have to randomly assign them. They’d have smaller tumors and lower event rates. Once we sat down and evaluated what kind of sample size we needed, such a trial would be difficult to conduct.
![]() DR LOVE: Have you used trastuzumab without chemotherapy?
DR LOVE: Have you used trastuzumab without chemotherapy?
![]() DR MUSS: No, I have not. A trial run by Dana-Farber, in which we’re
participating, is evaluating weekly paclitaxel and trastuzumab in the adjuvant
setting. We know that paclitaxel/trastuzumab is an effective combination in
the metastatic setting. Weekly paclitaxel has been a reasonably well-tolerated
chemotherapy, irrespective of age. I believe it’s a good design. It’s a modest
amount of chemotherapy — 12 cycles of weekly paclitaxel. Should you use
that outside of a clinical trial? I would administer something like TC with
trastuzumab as in the HERA trial if the patient were healthy enough.
DR MUSS: No, I have not. A trial run by Dana-Farber, in which we’re
participating, is evaluating weekly paclitaxel and trastuzumab in the adjuvant
setting. We know that paclitaxel/trastuzumab is an effective combination in
the metastatic setting. Weekly paclitaxel has been a reasonably well-tolerated
chemotherapy, irrespective of age. I believe it’s a good design. It’s a modest
amount of chemotherapy — 12 cycles of weekly paclitaxel. Should you use
that outside of a clinical trial? I would administer something like TC with
trastuzumab as in the HERA trial if the patient were healthy enough.
Track 16
![]() DR LOVE: How do you choose between lapatinib and trastuzumab or
both in terms of anti-HER2 therapy of HER2-positive metastatic disease?
DR LOVE: How do you choose between lapatinib and trastuzumab or
both in terms of anti-HER2 therapy of HER2-positive metastatic disease?
![]() DR MUSS: If the patient has metastatic breast cancer, does not have CNS
metastases and has never received trastuzumab, I use a trastuzumab-containing
combination. I’ve used vinorelbine, which I believe is a user-friendly drug.
It’s a little myelosuppressing, but otherwise it’s well tolerated. Or you can use
paclitaxel. I don’t know if a vast difference exists there.
DR MUSS: If the patient has metastatic breast cancer, does not have CNS
metastases and has never received trastuzumab, I use a trastuzumab-containing
combination. I’ve used vinorelbine, which I believe is a user-friendly drug.
It’s a little myelosuppressing, but otherwise it’s well tolerated. Or you can use
paclitaxel. I don’t know if a vast difference exists there.
If someone presented now with de novo brain metastases who had been on neither of the agents, quite honestly I’d probably pick lapatinib, and I might even consider something like capecitabine — which penetrates the CSF — along with it.
EDITOR'S NOTE
Austrian tag team
Neil Love, MD
- Select publications
INTERVIEWS
Michael Gnant, MD
- Select publications
Hyman B Muss, MD
- Select publications
Matthew J Ellis, MB, BChir, PhD
- Select publications
Charles L Vogel, MD (Fellows Rounds)
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity