
 |
||||||||
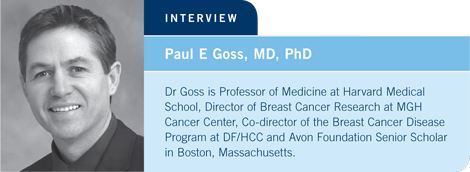
| Tracks 1-16 | ||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Do you have a theoretical explanation for the antitumor
activity observed with zoledronic acid in the ABCSG-12 trial presented at
the plenary session of ASCO?
DR LOVE: Do you have a theoretical explanation for the antitumor
activity observed with zoledronic acid in the ABCSG-12 trial presented at
the plenary session of ASCO?
![]() DR GOSS: One theory is that bone metabolism — the formation and resorption
of bone — engenders packages of nutrients, from which cancer cells in
the bone may benefit. Moreover, cancer cells located in metastases elsewhere may either receive the circulating nutrients or pass through the bone and
derive benefit from those nutritional growth factors. By slowing down bone
metabolism, the amount of nutrients available is decreased.
DR GOSS: One theory is that bone metabolism — the formation and resorption
of bone — engenders packages of nutrients, from which cancer cells in
the bone may benefit. Moreover, cancer cells located in metastases elsewhere may either receive the circulating nutrients or pass through the bone and
derive benefit from those nutritional growth factors. By slowing down bone
metabolism, the amount of nutrients available is decreased.
A parallel data set to the Austrian study presented by Allan Lipton at the 2007 San Antonio meeting precisely supported this theory (Lipton 2007, 2008; [1.1]). He evaluated breast cancer recurrence based on bone mineral density and demonstrated that patients who were the most osteoporotic or osteopenic had the highest risk of recurrence. Their data suggested that accelerated bone metabolism might engender the growth of micrometastatic cancer.
If that turns out to be true, it will be an interesting and important observation that ushers in the idea that the more quiescent bone is in the presence of cancer, the better patient outcomes will be. That is a broad, sweeping statement and may not be true for all types of breast cancer.
Track 4
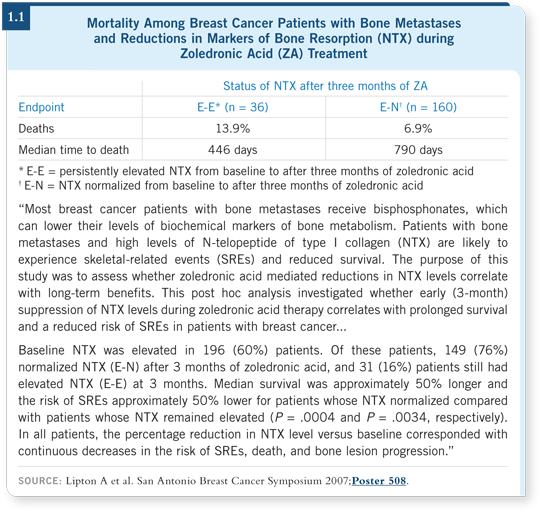
![]() DR LOVE: Based on the ABCSG data (1.2), do you believe that
zoledronic acid should be presented to patients as an option right now?
DR LOVE: Based on the ABCSG data (1.2), do you believe that
zoledronic acid should be presented to patients as an option right now?
![]() DR GOSS: Yes and no. We need to take some pause regarding the efficacy of
zoledronic acid in terms of the disease-free survival benefit because it was a
single, small trial. Fortunately, it can change practice in terms of bone preservation.
I don’t believe anyone will dispute that a premenopausal patient, regardless
of her ER status, who will have her ovarian functioning suppressed ought to
be treated preventively with bisphosphonate therapy because she will otherwise
lose an unacceptable amount of bone during the initial years of therapy.
DR GOSS: Yes and no. We need to take some pause regarding the efficacy of
zoledronic acid in terms of the disease-free survival benefit because it was a
single, small trial. Fortunately, it can change practice in terms of bone preservation.
I don’t believe anyone will dispute that a premenopausal patient, regardless
of her ER status, who will have her ovarian functioning suppressed ought to
be treated preventively with bisphosphonate therapy because she will otherwise
lose an unacceptable amount of bone during the initial years of therapy.
Is zoledronic acid the best bisphosphonate? Mechanistically, it is the most powerful, but do we need it to be that powerful? It may be important for premenopausal patients but not for postmenopausal patients. A Southwest Oncology Group trial (SWOG-S0307) is evaluating zoledronic acid versus ibandronate versus clodronate to determine which agent is the most effective.
Track 5
![]() DR LOVE: Would you discuss the long-term natural history of breast
cancer and the rationale for extended adjuvant endocrine therapy?
DR LOVE: Would you discuss the long-term natural history of breast
cancer and the rationale for extended adjuvant endocrine therapy?
![]() DR GOSS: The MA17 trial we conducted, extending adjuvant endocrine
therapy from five to 10 years with letrozole after tamoxifen, reflects the
chronicity of solid tumor malignancies in some patients and the opportunity
to interrupt the risk of late recurrences (Goss 2003).
DR GOSS: The MA17 trial we conducted, extending adjuvant endocrine
therapy from five to 10 years with letrozole after tamoxifen, reflects the
chronicity of solid tumor malignancies in some patients and the opportunity
to interrupt the risk of late recurrences (Goss 2003).
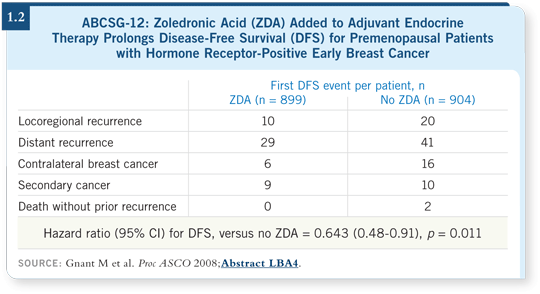
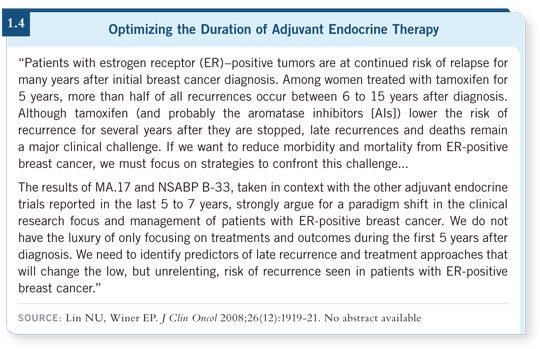
MA17 intrigued me because it was placebo controlled and provided an opportunity to study the natural event rate of breast cancer after five years of tamoxifen. Another important aspect of the study, recently published in the Journal of Clinical Oncology, was the observation that even if patients received a placebo for a while — one to seven years — after completion of five years of tamoxifen and then received delayed, extended letrozole, they still derived a profound proportional reduction in the risk of recurrence and death (Goss 2008a; [1.3]).
A simple message from this study is that a driver on cancer cells puts the patient at risk for recurrence and that driver may be present all the time, as reflected by an elevated annual hazard of recurrence that continues monotonously over time (1.4). We should attempt to interrupt that process if the risk is reasonably high and it is safe to do so.
Tracks 6-7
![]() DR LOVE: Can you discuss the TEACH trial, which is evaluating delayed
adjuvant treatment with lapatinib?
DR LOVE: Can you discuss the TEACH trial, which is evaluating delayed
adjuvant treatment with lapatinib?
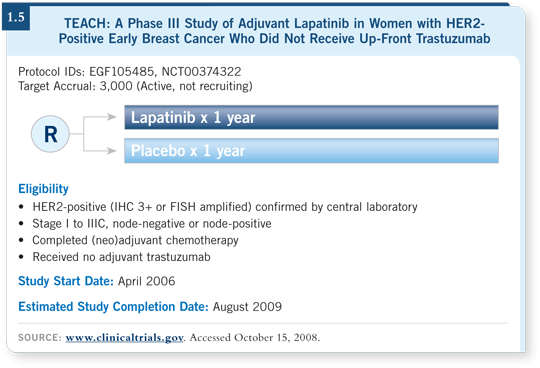
![]() DR GOSS: TEACH is a pragmatic clinical trial in which patients could be
randomly assigned to lapatinib or placebo for one year in a double-blinded
manner if they had not or could not receive adjuvant trastuzumab at diagnosis
for HER2-positive early breast cancer (1.5).
DR GOSS: TEACH is a pragmatic clinical trial in which patients could be
randomly assigned to lapatinib or placebo for one year in a double-blinded
manner if they had not or could not receive adjuvant trastuzumab at diagnosis
for HER2-positive early breast cancer (1.5).
The phrase “had not or could not” includes the rare patient in the United States with the up-front diagnosis of HER2-positive breast cancer who lives too far away to receive intravenous therapy or objects to the side effects of trastuzumab or for whatever reason will not be treated with adjuvant trastuzumab. In other countries, patients are unable to access trastuzumab for HER2-positive breast cancer.
We wanted at least 50 percent of the patients to be within four years of diagnosis, and we recently completed enrollment of about 3,200 patients. Approximately 80 percent of the patients are within four years of diagnosis — 20 percent are within one year, 60 percent are between one and four years — and another 20 percent were diagnosed four or more years ago.
In TEACH we have a marvelous opportunity to observe the natural history and the event rate across all subsets, including premenopausal and postmenopausal patients with ER-positive versus ER-negative and node-negative versus node-positive disease.
Track 10
![]() DR LOVE: What are your thoughts about the treatment of metastatic
breast cancer that progresses on chemotherapy and trastuzumab?
DR LOVE: What are your thoughts about the treatment of metastatic
breast cancer that progresses on chemotherapy and trastuzumab?
![]() DR GOSS: When patients with metastatic breast cancer experience disease
progression on chemotherapy and trastuzumab and could receive lapatinib/capecitabine, physicians struggle with whether to simply continue trastuzumab
and switch the chemotherapy.
DR GOSS: When patients with metastatic breast cancer experience disease
progression on chemotherapy and trastuzumab and could receive lapatinib/capecitabine, physicians struggle with whether to simply continue trastuzumab
and switch the chemotherapy.
In the original registration trial that led to the approval of lapatinib/capecitabine (Geyer 2006), a third investigational arm was glaringly absent, which was to continue the trastuzumab and administer capecitabine. Another idea is that perhaps a second anti-HER2 therapy could be added to trastuzumab (O’Shaughnessy 2008).
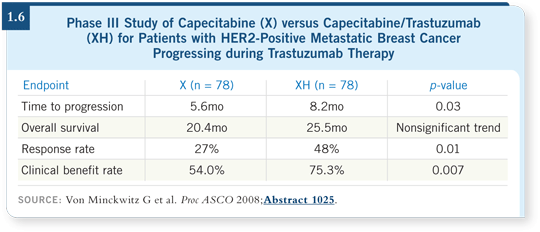
In another study with patients whose metastatic breast cancer was progressing on trastuzumab, trastuzumab was evaluated with the addition of capecitabine versus capecitabine alone (Von Minckwitz 2008; [1.6]). The superior strategy was to continue the trastuzumab. That study supported what doctors have been thinking but have been challenged on so strongly: “HER2 positivity is like fuel injection. You can change the chemotherapy but you must keep the cap on the fuel injection. Just because the cancer is progressing does not mean that the need to block the HER2 pathway diminishes.”
These data play against switching to lapatinib/capecitabine and may argue for switching chemotherapy but continuing trastuzumab and, eventually, possibly switching the anti-HER2 therapy.
Tracks 15-16
![]() DR LOVE: What are your thoughts about current trials evaluating
bevacizumab in the adjuvant setting?
DR LOVE: What are your thoughts about current trials evaluating
bevacizumab in the adjuvant setting?
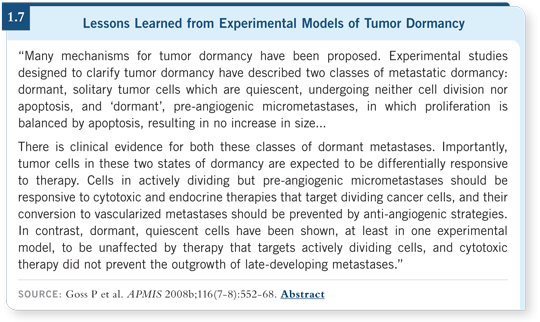
![]() DR GOSS: I strongly believe that the angiogenesis inhibitors will be important
in the adjuvant setting in a way that we haven’t observed in the metastatic setting. I recently published a paper with Ann Chambers from the University
of Western Ontario in Canada reviewing experimental approaches for
studying tumor dormancy and whether it offers a therapeutic target (Goss
2008b; [1.7]).
DR GOSS: I strongly believe that the angiogenesis inhibitors will be important
in the adjuvant setting in a way that we haven’t observed in the metastatic setting. I recently published a paper with Ann Chambers from the University
of Western Ontario in Canada reviewing experimental approaches for
studying tumor dormancy and whether it offers a therapeutic target (Goss
2008b; [1.7]).
To be simplistic, you can create a two-by-two dormancy table with quiescent or proliferative on one axis and prevascularized or vascularized on the other axis. Clinically, we potentially have four types of dormancy. You may have quiescent, prevascularized dormancy, which means no microvasculature and cells are not dividing, which is highly refractory to currently understood anticancer therapy. Patients with such tumors will not benefit from the concomitant administration of angiogenesis inhibitors or any type of antiproliferative therapy.
For the prevascularized, proliferative and the vascularized, proliferative types of dormancy, I believe that Judah Folkman’s pioneering concepts will turn out to be correct. Anti-angiogenic therapy will block recurrences of solid tumor malignancy profoundly because it will prevent that critical vascularization required for tumor growth. Dr Folkman postulated what many people have shown — the tumor can rest for a long time before it is capable of crossing a “magical” threshold to vascularization.
![]() DR LOVE: What about the other belief, that anti-angiogenic agents normalize
the tumor vasculature and allow better delivery of oxygen or chemotherapy?
DR LOVE: What about the other belief, that anti-angiogenic agents normalize
the tumor vasculature and allow better delivery of oxygen or chemotherapy?
![]() DR GOSS: That is an apparent contradiction, but I believe both theories are
correct. Is it prevention of vascularization that will inhibit tumor growth, or
is it the normalization of tumor vasculature that will allow therapy to be more
effectively delivered?
DR GOSS: That is an apparent contradiction, but I believe both theories are
correct. Is it prevention of vascularization that will inhibit tumor growth, or
is it the normalization of tumor vasculature that will allow therapy to be more
effectively delivered?
In a vascularized tumor, anti-angiogenic therapy may improve blood flow and enhance radiation therapy and the delivery of chemotherapy, or you can catch the micrometastases at that critical juncture when they’re beginning to develop microvasculature and stop that from happening at all. I believe we are seeing two different concepts of how the same therapy works. One addresses established cancer, with a rich but dysfunctional blood supply that impedes delivery of therapy, versus absolutely discouraging the development of both normal and abnormal vasculature, which is necessary to encourage tumor growth.
EDITOR
Neil Love, MD
Paul E Goss, MD, PhD
- Select publications
Martine J Piccart-Gebhart,
MD, PhD
- Select publications
TUMOR PANEL CASE DISCUSSION
- Select publications
INTERVIEWS (continued)
Erica L Mayer, MD, MPH
- Select publications
Maria Theodoulou, MD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity