
 |
||||||||

| Tracks 1-17 | ||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 2-3
![]() DR LOVE: How do you treat patients with smaller, node-negative,
HER2-positive tumors?
DR LOVE: How do you treat patients with smaller, node-negative,
HER2-positive tumors?
![]() DR PICCART-GEBHART: This is a real problem in daily clinical practice. Given
that these patients were not allowed to enter the adjuvant trials, we have no data.
I interviewed my colleagues and discovered that many do what I do, although
it’s based strictly on intuition. I am offering trastuzumab to women who have
tumors between five millimeters and one centimeter. For tumors smaller than
five millimeters, I am less comfortable with such a recommendation.
DR PICCART-GEBHART: This is a real problem in daily clinical practice. Given
that these patients were not allowed to enter the adjuvant trials, we have no data.
I interviewed my colleagues and discovered that many do what I do, although
it’s based strictly on intuition. I am offering trastuzumab to women who have
tumors between five millimeters and one centimeter. For tumors smaller than
five millimeters, I am less comfortable with such a recommendation.
So, although tumor size has clear prognostic significance, I still believe the biology is bad. You could argue that these women should only receive an anthracycline-based chemotherapy regimen. However, trastuzumab is such an elegant therapy.
![]() DR LOVE: Would you consider trastuzumab alone, without chemotherapy, for
those patients?
DR LOVE: Would you consider trastuzumab alone, without chemotherapy, for
those patients?
![]() DR PICCART-GEBHART: No, I would not do that presently because no data
exist for that approach either. You would be doing two things that are not at
all evidence based. For these women, I prefer the treatments that have been
tested.
DR PICCART-GEBHART: No, I would not do that presently because no data
exist for that approach either. You would be doing two things that are not at
all evidence based. For these women, I prefer the treatments that have been
tested.
![]() DR LOVE: Does ER status affect your decision with the smaller tumor?
DR LOVE: Does ER status affect your decision with the smaller tumor?
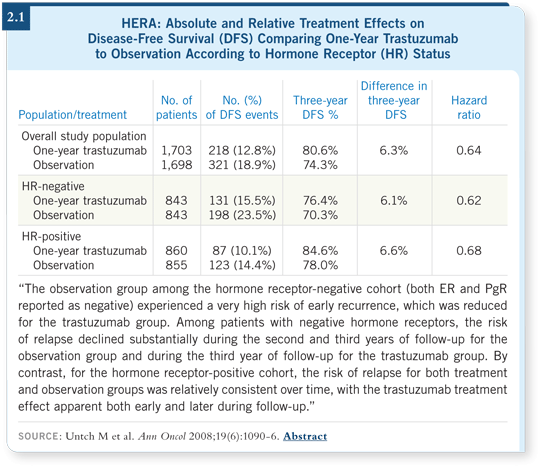
![]() DR PICCART-GEBHART: Not really. In the HERA trial, we saw a different
pattern of relapse in women who had ER-positive versus ER-negative tumors
(Untch 2008; [2.1]). The cancer in women with ER-negative disease tends
to recur early when they don’t receive trastuzumab. We observed this in the
control arm of the trial. For women with ER-positive disease who were not
receiving trastuzumab, we do not see an early peak of relapse. The relapse
rate also appears to be lower, although it could simply be a time effect. I don’t
believe we can make any treatment decision based on this observation. It could
be that the patients with ER-positive disease also relapse at a high rate, but it
happens a little later.
DR PICCART-GEBHART: Not really. In the HERA trial, we saw a different
pattern of relapse in women who had ER-positive versus ER-negative tumors
(Untch 2008; [2.1]). The cancer in women with ER-negative disease tends
to recur early when they don’t receive trastuzumab. We observed this in the
control arm of the trial. For women with ER-positive disease who were not
receiving trastuzumab, we do not see an early peak of relapse. The relapse
rate also appears to be lower, although it could simply be a time effect. I don’t
believe we can make any treatment decision based on this observation. It could
be that the patients with ER-positive disease also relapse at a high rate, but it
happens a little later.
Track 4
![]() DR LOVE: Can you discuss the issue of anthracycline-versus nonanthracycline-containing chemotherapy for node-positive, HER2-positive disease?
DR LOVE: Can you discuss the issue of anthracycline-versus nonanthracycline-containing chemotherapy for node-positive, HER2-positive disease?
![]() DR PICCART-GEBHART: That’s a hot topic. In Europe, we are selecting the
type of chemotherapy based on risk factors for cardiotoxicity, including age,
obesity, poorly controlled hypertension and a left ventricular ejection fraction
that is on the low end of the normal range prior to initiating therapy. For
patients who are at a higher risk for cardiotoxicity, it is reasonable to choose a
nonanthracycline-based chemotherapy.
DR PICCART-GEBHART: That’s a hot topic. In Europe, we are selecting the
type of chemotherapy based on risk factors for cardiotoxicity, including age,
obesity, poorly controlled hypertension and a left ventricular ejection fraction
that is on the low end of the normal range prior to initiating therapy. For
patients who are at a higher risk for cardiotoxicity, it is reasonable to choose a
nonanthracycline-based chemotherapy.
I stick to TCH, the regimen that has been piloted in the BCIRG 006 study (Slamon 2006). It is important to be able to clearly explain to patients the side effects they can expect with this regimen.
![]() DR LOVE: How would you treat a 38-year-old woman who has five positive
nodes and is otherwise perfectly healthy?
DR LOVE: How would you treat a 38-year-old woman who has five positive
nodes and is otherwise perfectly healthy?
![]() DR PICCART-GEBHART: We have two options. The five positive nodes are
worrisome and indicate a higher risk for an early relapse. You do not want to
administer a six-month chemotherapy regimen and then start trastuzumab.
It makes sense for such a woman to receive TCH or utilize our European
approach, which is three cycles of FEC — this is anthracycline based but only
three cycles — and then move on to a taxane, which can be docetaxel or
paclitaxel, administered concomitantly with trastuzumab.
DR PICCART-GEBHART: We have two options. The five positive nodes are
worrisome and indicate a higher risk for an early relapse. You do not want to
administer a six-month chemotherapy regimen and then start trastuzumab.
It makes sense for such a woman to receive TCH or utilize our European
approach, which is three cycles of FEC — this is anthracycline based but only
three cycles — and then move on to a taxane, which can be docetaxel or
paclitaxel, administered concomitantly with trastuzumab.
Tracks 5-6
![]() DR LOVE: Can you discuss the ALTTO adjuvant trial?
DR LOVE: Can you discuss the ALTTO adjuvant trial?
![]() DR PICCART-GEBHART: When we design these trials, we have a responsibility
to ask interesting questions. With the ALTTO study, we felt that it was important
to compare the relative merits of two anti-HER2 treatments (2.2).
DR PICCART-GEBHART: When we design these trials, we have a responsibility
to ask interesting questions. With the ALTTO study, we felt that it was important
to compare the relative merits of two anti-HER2 treatments (2.2).
The single-agent lapatinib arm has not made everyone comfortable, but we believe that the lapatinib data in metastatic breast cancer are encouraging. It is important to examine what a small molecule administered orally can do, as opposed to an antibody that must be administered in a hospital setting. In some countries in the world, it might be a problem to go to the hospital every three weeks for a full year.
The other arms are exciting. One of them is exploring the sequence of the two drugs — three months of trastuzumab, a short washout period and then lapatinib to complete a year of treatment. The third arm, which could be the winner, is the combination of the two agents.
Chemotherapy can be introduced in two ways. One is to administer the chemotherapy first, and you have a lot of flexibility in the choice of chemotherapy regimen. The second option is the concurrent administration of the biologics with paclitaxel. Paclitaxel was chosen because the only safety data available at this time are with paclitaxel. We will probably introduce an option for docetaxel in the near future because we are beginning to see data there.
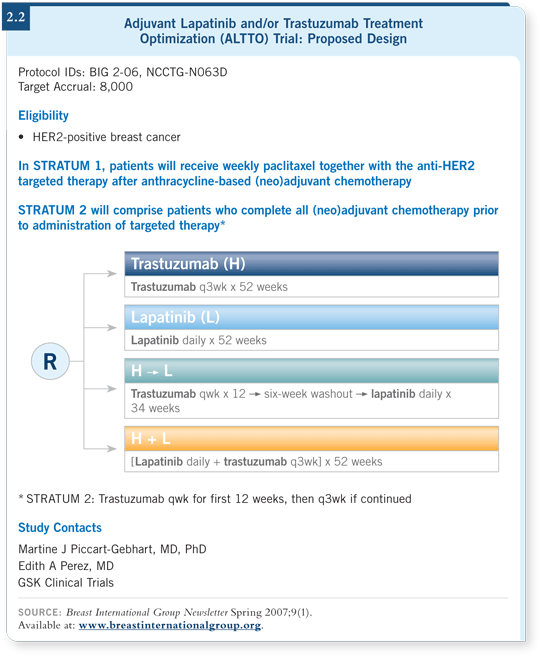
Currently, the ALTTO trial is requiring patients to receive anthracyclines, but this may also change. We don’t want to be in a situation when the trial is finished in which people tell us anthracyclines are no longer needed. We are hoping to be able to allow regimens such as TCH to be used in the trial.
![]() DR LOVE: What do we know about the safety of the paclitaxel/lapatinib
combination and the combination of paclitaxel with lapatinib/trastuzumab?
DR LOVE: What do we know about the safety of the paclitaxel/lapatinib
combination and the combination of paclitaxel with lapatinib/trastuzumab?
![]() DR PICCART-GEBHART: Initially we chose the doses on limited data, which
now have been expanded. It has become apparent that some women experience
severe diarrhea with the doses we initially selected. Although it is
possible to manage this type of toxicity in sophisticated cancer centers, this
trial should mean something to practices all around the world.
DR PICCART-GEBHART: Initially we chose the doses on limited data, which
now have been expanded. It has become apparent that some women experience
severe diarrhea with the doses we initially selected. Although it is
possible to manage this type of toxicity in sophisticated cancer centers, this
trial should mean something to practices all around the world.
We made the decision to reduce the dose of lapatinib to 750 milligrams instead of the 1,000-mg dose, but we did not touch the dose of paclitaxel. We know from experiences in two cancer centers, Memorial Sloan-Kettering (Dang 2008) and the Mayo Clinic in Florida, that when you reduce the dose of lapatinib, you can continue with the treatment. We assume that if we start with this lower dose, the toxicity will be acceptable. We will monitor patients extremely closely in the adjuvant study. We hope that this is not going to compromise efficacy, but we could not run the risk of toxic deaths in a study such as the ALTTO trial.
Tracks 11-12
![]() DR LOVE: Can you discuss the main findings of the ABCSG-12 study?
DR LOVE: Can you discuss the main findings of the ABCSG-12 study?
![]() DR PICCART-GEBHART: This is a fascinating trial. If the results can be duplicated
in a second study, I believe we will be entering a new era in the fight
against the disease. The focus will shift away from the tumor toward the
importance of the host.
DR PICCART-GEBHART: This is a fascinating trial. If the results can be duplicated
in a second study, I believe we will be entering a new era in the fight
against the disease. The focus will shift away from the tumor toward the
importance of the host.
We have to remember that ABCSG-12 was highly selective with its entry criteria (Gnant 2008). It was a trial for premenopausal women with endocrine-responsive breast cancer whose physicians were comfortable not administering chemotherapy. The first randomization was between two endocrine treatment strategies. The second randomization was to zoledronic acid every six months or placebo.
![]() DR LOVE: A fair number of patients with node-positive disease were enrolled
on this study, yet the five-year relapse rate was approximately six percent,
which is striking.
DR LOVE: A fair number of patients with node-positive disease were enrolled
on this study, yet the five-year relapse rate was approximately six percent,
which is striking.
![]() DR PICCART-GEBHART: That’s not so surprising to Europeans because we have
been defending endocrine therapy for a long time. For women who do not
have massive nodal involvement and whose tumor is highly endocrine responsive,
we are convinced that the benefit of chemotherapy is small or nonexistent.
DR PICCART-GEBHART: That’s not so surprising to Europeans because we have
been defending endocrine therapy for a long time. For women who do not
have massive nodal involvement and whose tumor is highly endocrine responsive,
we are convinced that the benefit of chemotherapy is small or nonexistent.
We have to remember that this was not a broad population but a highly selected population. Therefore, we have to wait for confirmation from one of the other trials that has examined a larger population. When I evaluated all the preclinical work during the past 10 years that exists on bisphosphonates, particularly zoledronic acid, I began to believe that these drugs might have potential beyond ER-positive breast cancer.
I am optimistic. I believe the AZURE trial will confirm these data. AZURE is a trial for patients with node-positive disease that can be ER-positive or ER-negative. It includes younger and older women, so it will be more representative of the breast cancer population. The bisphosphonate is also administered in a more intensive fashion. If this trial is positive, I believe this agent will become a standard treatment.
![]() DR LOVE: ABCSG-12 reported a striking 35 percent decrease in relapse rate
(Gnant 2008). What was interesting was that it wasn’t only in bone. It was in
contralateral primary tumors and metastatic disease. Can you talk about the seed
and soil hypothesis that you presented in your ASCO discussion of this paper?
DR LOVE: ABCSG-12 reported a striking 35 percent decrease in relapse rate
(Gnant 2008). What was interesting was that it wasn’t only in bone. It was in
contralateral primary tumors and metastatic disease. Can you talk about the seed
and soil hypothesis that you presented in your ASCO discussion of this paper?
![]() DR PICCART-GEBHART: This requires that we speculate about breast cancer
progression models. The seed and soil hypothesis is that breast cancer cells
leave the breast much earlier than we think, find a niche in bone and from
there metastasize to other organs and even go back to the initial site where
they came from in the breast.
DR PICCART-GEBHART: This requires that we speculate about breast cancer
progression models. The seed and soil hypothesis is that breast cancer cells
leave the breast much earlier than we think, find a niche in bone and from
there metastasize to other organs and even go back to the initial site where
they came from in the breast.
We think this occurs only in a certain type of patient. It is similar to what Larry Norton has been showing recently (Norton 2006), even before knowing about the results of the Austrian trial.
The ABCSG-12 trial is challenging our belief about this stepwise progression model, in which the cancer cell is first in the breast and then travels to the nodes and elsewhere. Maybe that’s not at all what is happening in the real world.
Track 13
![]() DR LOVE: In your ASCO discussion, you cautioned people about translating
these data into practice. Right now, do you discuss this with your
patients as a possibility?
DR LOVE: In your ASCO discussion, you cautioned people about translating
these data into practice. Right now, do you discuss this with your
patients as a possibility?
![]() DR PICCART-GEBHART: I am talking to my patients who are in exactly the
scenario of the Austrian study — for example, patients whom I am treating
with goserelin and tamoxifen. I’ve been telling my patients that I am waiting
for the presentation of a second study, I hope by the end of this year.
DR PICCART-GEBHART: I am talking to my patients who are in exactly the
scenario of the Austrian study — for example, patients whom I am treating
with goserelin and tamoxifen. I’ve been telling my patients that I am waiting
for the presentation of a second study, I hope by the end of this year.
So I’ve scheduled appointments for these patients for the beginning of 2009, and I’ve asked them to go to a dentist beforehand for a baseline evaluation because of the small risk of osteonecrosis of the jaw (ONJ). Although ONJ was not observed in the Austrian study, we know that it is a potential complication of bisphosphonate therapy.
Again, I don’t know what will happen at the beginning of 2009, but if the second study is positive, then it’s an easy decision. We will start administering this agent to patients. If AZURE is not reported at San Antonio, then women have to make a decision for themselves after the full explanation of potential benefits and risks.
Track 16
![]() DR LOVE: What are your thoughts about continuation of hormonal
therapy for postmenopausal patients who have received five years of an
aromatase inhibitor?
DR LOVE: What are your thoughts about continuation of hormonal
therapy for postmenopausal patients who have received five years of an
aromatase inhibitor?
![]() DR PICCART-GEBHART: I belong to the group of people who view hormone
receptor-positive breast cancer as a disease that might be difficult to cure and
might require lifelong treatment. If we are able to conduct good translational
research in some of the big endocrine therapy trials, and if we are able to
complete pharmacogenetic studies, we might be able to identify those patients
who are at risk for long-term relapses.
DR PICCART-GEBHART: I belong to the group of people who view hormone
receptor-positive breast cancer as a disease that might be difficult to cure and
might require lifelong treatment. If we are able to conduct good translational
research in some of the big endocrine therapy trials, and if we are able to
complete pharmacogenetic studies, we might be able to identify those patients
who are at risk for long-term relapses.
Perhaps not all women with hormone receptor-positive breast cancer are at risk for long-term relapses, but right now, we don’t have a way to identify who is at risk and who is not. In our practice, we observe women who have these relapses occurring nine, 12, 15 years after initial therapy.
If you believe in the long-term risk, then stopping an aromatase inhibitor after five years doesn’t make a lot of sense. I am trying to continue treatment for up to seven, eight, sometimes 10 years. I recognize that we have few data, but it’s a question of philosophy and how you view this disease. Unfortunately, this is a disease that probably has to be viewed as a chronic one, requiring continuous endocrine manipulation.
EDITOR
Neil Love, MD
Paul E Goss, MD, PhD
- Select publications
Martine J Piccart-Gebhart,
MD, PhD
- Select publications
TUMOR PANEL CASE DISCUSSION
- Select publications
INTERVIEWS (continued)
Erica L Mayer, MD, MPH
- Select publications
Maria Theodoulou, MD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity