
 |
||||||||

| Tracks 1-23 | ||||||||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1, 4-5

![]() DR LOVE: How do you generally approach the care of patients with
subcentimeter, node-negative, HER2-positive tumors?
DR LOVE: How do you generally approach the care of patients with
subcentimeter, node-negative, HER2-positive tumors?
![]() DR MACKEY: For patients with these small tumors, we do not have proof of
benefit from adjuvant trastuzumab. The adjuvant trastuzumab trials didn’t
include patients with tumors this small. At the end of the day, we have no
randomized trial evidence suggesting this would be of benefit. And, unfortunately,
with an effective drug such as trastuzumab, it has to be combined
with chemotherapy in the adjuvant setting — at least that’s where we have the
evidence.
DR MACKEY: For patients with these small tumors, we do not have proof of
benefit from adjuvant trastuzumab. The adjuvant trastuzumab trials didn’t
include patients with tumors this small. At the end of the day, we have no
randomized trial evidence suggesting this would be of benefit. And, unfortunately,
with an effective drug such as trastuzumab, it has to be combined
with chemotherapy in the adjuvant setting — at least that’s where we have the
evidence.
![]() DR GRALOW: This is a tough situation because I believe trastuzumab has
potential to add benefit. I don’t know how much this benefit is dependent
upon the synergy with chemotherapy. Within the Southwest Oncology Group,
we’ve been talking about aromatase inhibitors with a HER2-targeted agent,
at least in an ER-positive, HER2-positive setting.
DR GRALOW: This is a tough situation because I believe trastuzumab has
potential to add benefit. I don’t know how much this benefit is dependent
upon the synergy with chemotherapy. Within the Southwest Oncology Group,
we’ve been talking about aromatase inhibitors with a HER2-targeted agent,
at least in an ER-positive, HER2-positive setting.
We will be participating in a trial evaluating paclitaxel with trastuzumab in a group of patients with node-negative disease who have otherwise good risk features. We’ll knock out the anthracycline, and weekly paclitaxel is less toxic than docetaxel/carboplatin. We struggle, however, with the thought that if we’re not using chemotherapy, are we providing as much benefit from trastuzumab? If you send out for the Oncotype DX 21-gene Recurrence Score, these patients always fall in the high-risk category.
![]() DR LOVE: Hope, what would you expect from hormonal therapy for a patient
with ER-positive, HER2-positive disease?
DR LOVE: Hope, what would you expect from hormonal therapy for a patient
with ER-positive, HER2-positive disease?
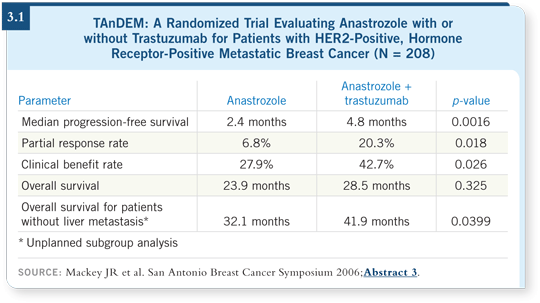
![]() DR RUGO: We don’t have a whole lot of data. In the trial that randomly
assigned patients with ER-positive, HER2-positive, hormone therapy-naïve
metastatic disease to anastrozole with or without trastuzumab, the response
rate for anastrozole was not particularly high and the duration of response was
particularly short at only 2.4 months. Even when trastuzumab was added, the
results weren’t fabulous, although they were better than with anastrozole alone
(Mackey 2006; [3.1]).
DR RUGO: We don’t have a whole lot of data. In the trial that randomly
assigned patients with ER-positive, HER2-positive, hormone therapy-naïve
metastatic disease to anastrozole with or without trastuzumab, the response
rate for anastrozole was not particularly high and the duration of response was
particularly short at only 2.4 months. Even when trastuzumab was added, the
results weren’t fabulous, although they were better than with anastrozole alone
(Mackey 2006; [3.1]).
I believe, however, that the adjuvant setting is different. For patients with low-risk disease, hormonal therapy may be important. We don’t have data indicating that tamoxifen is not effective for that population. It simply isn’t as effective as it is for patients with HER2-normal disease. I would treat a patient with HER2-positive disease as I would treat a patient with HER2-negative disease and use hormonal therapy. The bigger question is, do you add chemotherapy with or without trastuzumab?
![]() DR LOVE: Julie, what about your trial for this patient, SWOG-S0307?
DR LOVE: Julie, what about your trial for this patient, SWOG-S0307?
![]() DR GRALOW: SWOG-S0307 is comparing three different bisphosphonates.
We’re using clodronate as our standard arm. The comparators are three years
of oral ibandronate versus three years of a dose-intensive zoledronic acid
regimen that is administered monthly for six months and then on an every
three-month schedule.
DR GRALOW: SWOG-S0307 is comparing three different bisphosphonates.
We’re using clodronate as our standard arm. The comparators are three years
of oral ibandronate versus three years of a dose-intensive zoledronic acid
regimen that is administered monthly for six months and then on an every
three-month schedule.
This patient would be a candidate for SWOG-S0307. The patients have to be receiving some form of systemic treatment, either hormonal therapy or chemotherapy. Trastuzumab alone wouldn’t be sufficient for enrollment in this study. If, for whatever reason, she weren’t eligible or declined participation in the trial, the question would be whether she would fit the criteria for the less intensive every six-month zoledronic acid regimen used in ABCSG-12 (Gnant 2008).
ABCSG-12 enrolled a population of premenopausal women who received endocrine therapy but not chemotherapy (Gnant 2008). This patient fits these criteria, although that group also received ovarian suppression. Something about ovarian suppression and shutting off estrogen and more rapid bone loss may be occurring in that study. I believe, however, she’s one of the small percentage of patients with breast cancer who meet the criteria for that study. It would be reasonable to talk with her about the results from ABCSG-12.
![]() DR LOVE: Hope, would you offer zoledronic acid to this patient?
DR LOVE: Hope, would you offer zoledronic acid to this patient?
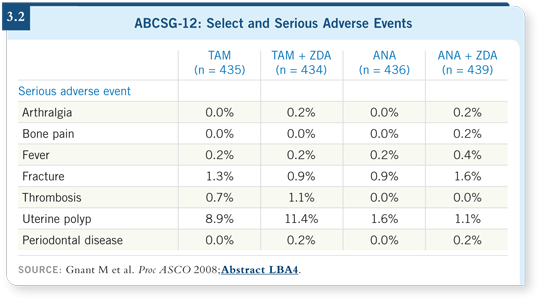
![]() DR RUGO: I believe the data from the ABCSG-12 trial were impressive, and
the toxicity was modest (Gnant 2008; [3.2]). They had few events in either arm, however, so it’s a little early for me. We don’t have a labeled indication
for zoledronic acid every six months, and we haven’t used it off study.
However, I have encouraged patients to enroll on SWOG-S0307. I’m fascinated
with the fact that patients are hesitant because of this huge flurry in the
lay press about ONJ.
DR RUGO: I believe the data from the ABCSG-12 trial were impressive, and
the toxicity was modest (Gnant 2008; [3.2]). They had few events in either arm, however, so it’s a little early for me. We don’t have a labeled indication
for zoledronic acid every six months, and we haven’t used it off study.
However, I have encouraged patients to enroll on SWOG-S0307. I’m fascinated
with the fact that patients are hesitant because of this huge flurry in the
lay press about ONJ.
![]() DR GRALOW: In SWOG-S0307, we have one documented case of ONJ in the
500 patients enrolled on the zoledronic acid arm. In the AZURE trial, approximately
1,500 patients have received zoledronic acid, and we’ve seen seven
cases of ONJ. I would say that ONJ is a real entity. In the AZURE trial, they
weren’t proactive about oral/dental screening. We are not excluding anybody
based on their oral health, but we are mandating a baseline dental exam so that
we can monitor risk factors.
DR GRALOW: In SWOG-S0307, we have one documented case of ONJ in the
500 patients enrolled on the zoledronic acid arm. In the AZURE trial, approximately
1,500 patients have received zoledronic acid, and we’ve seen seven
cases of ONJ. I would say that ONJ is a real entity. In the AZURE trial, they
weren’t proactive about oral/dental screening. We are not excluding anybody
based on their oral health, but we are mandating a baseline dental exam so that
we can monitor risk factors.
Tracks 7-11

![]() DR LOVE: Hope, which treatment options would you have considered for
this patient?
DR LOVE: Hope, which treatment options would you have considered for
this patient?
![]() DR RUGO: I believe you need to balance whether to treat this patient with a
taxane/bevacizumab-type approach or something else that might not produce
hair loss, for which I believe most of us would choose a capecitabine-type
approach. I don’t combine capecitabine with bevacizumab because I don’t
believe we have sufficient data with that treatment approach yet.
DR RUGO: I believe you need to balance whether to treat this patient with a
taxane/bevacizumab-type approach or something else that might not produce
hair loss, for which I believe most of us would choose a capecitabine-type
approach. I don’t combine capecitabine with bevacizumab because I don’t
believe we have sufficient data with that treatment approach yet.
I would offer her, if she had significant liver metastases, a paclitaxel/bevacizumab-type approach first. If she said, “The most important thing to me is to keep my hair intact,” then I’d start with capecitabine.
![]() DR LOVE: If you were going to use paclitaxel, would you use nab paclitaxel?
DR LOVE: If you were going to use paclitaxel, would you use nab paclitaxel?
![]() DR RUGO: For a patient who received paclitaxel two and a half years ago, we
generally use paclitaxel. If the patient experienced toxicity with the weekly
steroids, then I would make the case to use nab paclitaxel as a first-line
approach. In fact, I believe most of us prefer using nab paclitaxel if we can. For
a patient who’s received prior paclitaxel, I’m comfortable using nab paclitaxel
and avoiding the steroids, which I think markedly improves the tolerance to
therapy.
DR RUGO: For a patient who received paclitaxel two and a half years ago, we
generally use paclitaxel. If the patient experienced toxicity with the weekly
steroids, then I would make the case to use nab paclitaxel as a first-line
approach. In fact, I believe most of us prefer using nab paclitaxel if we can. For
a patient who’s received prior paclitaxel, I’m comfortable using nab paclitaxel
and avoiding the steroids, which I think markedly improves the tolerance to
therapy.
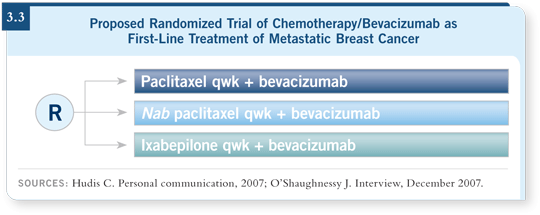
In the next few months, we will begin a first-line Phase III randomized trial, a collaboration between CALGB and NCCTG, that randomly assigns women with chemotherapy-naïve metastatic disease to paclitaxel, nab paclitaxel or ixabepilone, and all patients receive bevacizumab as well. Patients will receive weekly therapy three out of every four weeks (3.3).
![]() DR LOVE: How did this patient fare with the premedication with steroids in
the adjuvant setting?
DR LOVE: How did this patient fare with the premedication with steroids in
the adjuvant setting?
![]() DR GRALOW: She didn’t have any major problem with the steroids. I would
prefer nab paclitaxel because you don’t need steroids and antihistamines. I can
probably use the drug at a somewhat higher dose, with at least randomized
Phase II data suggesting more efficacy. Also, it’s a shorter infusion time generally.
So, if I can obtain insurance approval, certainly in the metastatic setting,
it would be my preference.
DR GRALOW: She didn’t have any major problem with the steroids. I would
prefer nab paclitaxel because you don’t need steroids and antihistamines. I can
probably use the drug at a somewhat higher dose, with at least randomized
Phase II data suggesting more efficacy. Also, it’s a shorter infusion time generally.
So, if I can obtain insurance approval, certainly in the metastatic setting,
it would be my preference.
Tracks 16-19

![]() DR LOVE: John, what would you recommend for systemic therapy for this
patient?
DR LOVE: John, what would you recommend for systemic therapy for this
patient?
![]() DR MACKEY: Technically, she has node-positive, HER2-negative disease and
is in good health at age 65. In this case, we’d be offering chemotherapy as
an option and hormonal therapy as a component of treatment. The chemotherapy
we’d discuss for women with fewer than three positive nodes would
be docetaxel/cyclophosphamide (TC). So we’d recommend four cycles of TC
and discuss an aromatase inhibitor.
DR MACKEY: Technically, she has node-positive, HER2-negative disease and
is in good health at age 65. In this case, we’d be offering chemotherapy as
an option and hormonal therapy as a component of treatment. The chemotherapy
we’d discuss for women with fewer than three positive nodes would
be docetaxel/cyclophosphamide (TC). So we’d recommend four cycles of TC
and discuss an aromatase inhibitor.
![]() DR GRALOW: I agree that chemotherapy should be recommended in this
case. In the TAILORx trial, she would clearly fall in the range where chemotherapy
would be used. Considering the high Recurrence Score, I would
probably favor an anthracycline/taxane-containing regimen as long as she had
good cardiac function.
DR GRALOW: I agree that chemotherapy should be recommended in this
case. In the TAILORx trial, she would clearly fall in the range where chemotherapy
would be used. Considering the high Recurrence Score, I would
probably favor an anthracycline/taxane-containing regimen as long as she had
good cardiac function.
Off study, I like dose-dense AC![]() paclitaxel. I would talk with her about
clinical trials, though. I believe it would be reasonable to offer her participation
in the ongoing trial of AC versus paclitaxel (CALGB-40101). I believe
that patients with high Recurrence Scores have chemotherapy-responsive
disease, and the manipulations we make to obtain a higher response benefit
these patients the most.
paclitaxel. I would talk with her about
clinical trials, though. I believe it would be reasonable to offer her participation
in the ongoing trial of AC versus paclitaxel (CALGB-40101). I believe
that patients with high Recurrence Scores have chemotherapy-responsive
disease, and the manipulations we make to obtain a higher response benefit
these patients the most.
We use a lot of chemotherapy in patients who don’t derive a lot of benefit from it, and the difference between CMF and dose-dense AC is not as great for those patients. She has a high Recurrence Score, however, suggesting a lot of potential benefit from chemotherapy.
![]() DR RUGO: For true node-positive disease, we tend to use dose-dense AC
DR RUGO: For true node-positive disease, we tend to use dose-dense AC![]() T
or suggest a clinical trial. I’ve found patients to be fairly responsive to participating
in ECOG-E5103, the bevacizumab trial, and less so to the AC versus
paclitaxel trial (CALGB-40101).
T
or suggest a clinical trial. I’ve found patients to be fairly responsive to participating
in ECOG-E5103, the bevacizumab trial, and less so to the AC versus
paclitaxel trial (CALGB-40101).
In a 65-year-old patient with ER/PR-positive disease who has minimal disease in the nodes, I feel comfortable offering TC. She has a high Recurrence Score, which is a bit unusual. We went back and rechecked her HER2 status, and I called Genomic Health to find out if her HER2 status fell into the positive range, but it didn’t.
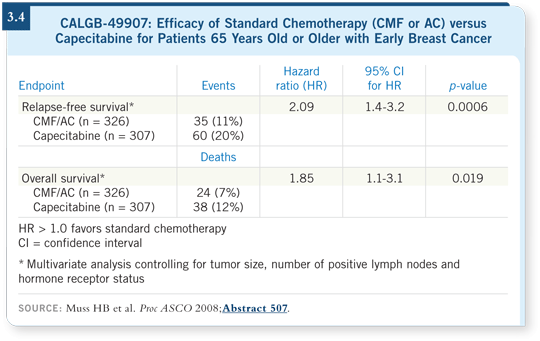
In this particular situation, I would discuss both regimens with the patient and get a sense from the patient about how aggressive she wanted to be. I feel comfortable using TC as a regimen. Based on Hy Muss’s presentation on elderly patients, evaluating capecitabine versus the physician’s choice of AC or CMF (Muss 2008; [3.4]), I believe CMF is a reasonable option.
I would also encourage this patient to take an aromatase inhibitor. However, we have to keep in mind that this patient would also benefit from tamoxifen. Some patients tolerate tamoxifen better over time. So I believe either the switching approach or an aromatase inhibitor up front would be reasonable. We tend to use the aromatase inhibitor up front. She’s also a candidate for SWOG-S0307, the bisphosphonate trial.
![]() DR LOVE: John, what are your thoughts about using the Oncotype DX assay
for node-positive disease?
DR LOVE: John, what are your thoughts about using the Oncotype DX assay
for node-positive disease?
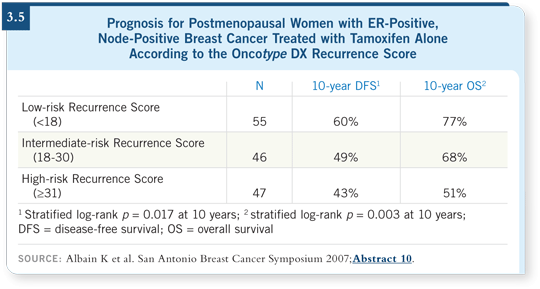
![]() DR MACKEY: We don’t use the Oncotype DX assay for patients with node-positive
disease. The data presented by Kathy Albain are interesting and
encouraging, but if you view the relapse rate for the women who received
tamoxifen alone, even if they had a low Recurrence Score, a substantial
number of recurrences still occurred (Albain 2007; [3.5]). So I don’t believe
that the omission of chemotherapy for patients with node-positive disease is
fully established, based on any molecular marker.
DR MACKEY: We don’t use the Oncotype DX assay for patients with node-positive
disease. The data presented by Kathy Albain are interesting and
encouraging, but if you view the relapse rate for the women who received
tamoxifen alone, even if they had a low Recurrence Score, a substantial
number of recurrences still occurred (Albain 2007; [3.5]). So I don’t believe
that the omission of chemotherapy for patients with node-positive disease is
fully established, based on any molecular marker.
![]() DR GRALOW: I feel most comfortable using the Oncotype DX assay in patients
with only a little disease in the nodes, although I’m not sure that’s where we’ll
end up. We are able to identify a group of patients with relatively chemotherapy-resistant disease. I agree entirely that one of the most important
aspects of the study is that the group with positive nodes and low Recurrence
Scores don’t fare well and we need to do better.
DR GRALOW: I feel most comfortable using the Oncotype DX assay in patients
with only a little disease in the nodes, although I’m not sure that’s where we’ll
end up. We are able to identify a group of patients with relatively chemotherapy-resistant disease. I agree entirely that one of the most important
aspects of the study is that the group with positive nodes and low Recurrence
Scores don’t fare well and we need to do better.
We’re struggling with the successor to that analysis. We’ve tried to add patients with node-positive disease to the ongoing TAILORx trial, but it was rejected outright by CTEP. We’re considering a trial in which we will add biologic agents or use manipulations of the endocrine therapy for this group.
If this is the group with disease that is sensitive to endocrine therapy, maybe we should be asking additional endocrine questions, such as whether to add fulvestrant to an aromatase inhibitor. I feel comfortable omitting chemotherapy for a patient like this if her Recurrence Score is low because chemotherapy won’t add benefit. I don’t, however, feel comfortable telling her that she will have a terrific survival rate.
EDITOR
Neil Love, MD
Paul E Goss, MD, PhD
- Select publications
Martine J Piccart-Gebhart,
MD, PhD
- Select publications
TUMOR PANEL CASE DISCUSSION
- Select publications
INTERVIEWS (continued)
Erica L Mayer, MD, MPH
- Select publications
Maria Theodoulou, MD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity