
 |
||||||||

| Tracks 1-8 | ||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Would you comment on the issue of capecitabine dose and
schedule?
DR LOVE: Would you comment on the issue of capecitabine dose and
schedule?
![]() DR THEODOULOU: It’s interesting because, although capecitabine is a popular
drug, a large dropout rate exists among patients attempting to adhere to the
standard 2,500 mg/m2 divided in two doses, 14 days on and seven days off.
If we can evaluate an efficacious way of administering capecitabine in which
we wouldn’t compromise its ability to kill cells yet minimize toxicity, then I
suspect patients could be treated for a much longer period.
DR THEODOULOU: It’s interesting because, although capecitabine is a popular
drug, a large dropout rate exists among patients attempting to adhere to the
standard 2,500 mg/m2 divided in two doses, 14 days on and seven days off.
If we can evaluate an efficacious way of administering capecitabine in which
we wouldn’t compromise its ability to kill cells yet minimize toxicity, then I
suspect patients could be treated for a much longer period.
It has been my practice not to rule out a regimen if patients do not tolerate it well but to make regimen adjustments instead with the goal of trying to home in on maintaining a clinical benefit while also minimizing toxicity. For some patients, I’ve been able to achieve a 10-day-on, seven-day-off treatment regimen, whereas for others I dose reduce. I’ve had about two dozen patients at any one time taking capecitabine, and they were all on different regimens with different tolerability.
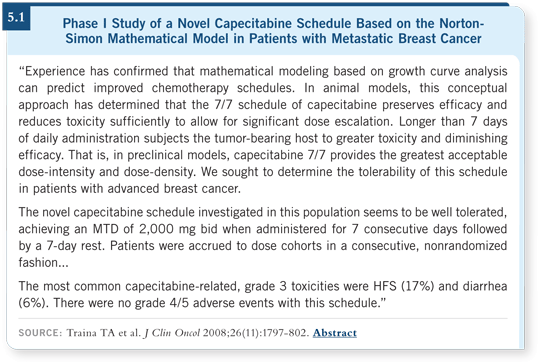
Our group at Memorial has been fairly successful in evaluating capecitabine dose and schedule. We’ve recently published some Phase I results in the Journal of Clinical Oncology clearly demonstrating feasibility with the capecitabine schedule of one week on followed by one week off (Traina 2008; [5.1]). Capecitabine is now being combined with bevacizumab in non-HER2 overexpressing breast cell lines in a study that’s about two thirds of the way toward completing accrual, and we’re moving forward with opening a protocol evaluating capecitabine biweekly with lapatinib.
Track 3
![]() DR LOVE: What’s your current algorithm for chemotherapy usage off
study in the metastatic setting for a patient with ER-positive/HER2-negative disease who is not responding to hormonal therapy?
DR LOVE: What’s your current algorithm for chemotherapy usage off
study in the metastatic setting for a patient with ER-positive/HER2-negative disease who is not responding to hormonal therapy?
![]() DR THEODOULOU: For those patients I prefer to use a bevacizumab-related
regimen as early as possible, either trying to capture them first line, as in the
studies reported recently by Kathy Miller and David Miles (Miller 2007; Miles
2008), or if they have been treated with other chemotherapy agents, trying
to capture them as early on as possible. We don’t have positive survival data
currently with bevacizumab, but the response rates and the time to progression
have been impressive.
DR THEODOULOU: For those patients I prefer to use a bevacizumab-related
regimen as early as possible, either trying to capture them first line, as in the
studies reported recently by Kathy Miller and David Miles (Miller 2007; Miles
2008), or if they have been treated with other chemotherapy agents, trying
to capture them as early on as possible. We don’t have positive survival data
currently with bevacizumab, but the response rates and the time to progression
have been impressive.
![]() DR LOVE: Are you using bevacizumab with capecitabine off study?
DR LOVE: Are you using bevacizumab with capecitabine off study?
![]() DR THEODOULOU: We’re evaluating bevacizumab with biweekly capecitabine
in our clinical trial. But if I have a choice off study currently, I administer
bevacizumab with a taxane first. For a patient with indolent, minimal-burden disease, I often use capecitabine as a single agent after anthracycline/taxane failures in the non-HER2 setting. If a patient’s disease progresses on
capecitabine, then I’ll consider bevacizumab with a taxane.
DR THEODOULOU: We’re evaluating bevacizumab with biweekly capecitabine
in our clinical trial. But if I have a choice off study currently, I administer
bevacizumab with a taxane first. For a patient with indolent, minimal-burden disease, I often use capecitabine as a single agent after anthracycline/taxane failures in the non-HER2 setting. If a patient’s disease progresses on
capecitabine, then I’ll consider bevacizumab with a taxane.
I’m a big believer in quality of life and gentle treatment. “Innocent-bystander” organ toxicity is an important issue. Most of these patients become like family because they’ve been around so long, thankfully, and we are treating them for a long time. We evaluate their goals together for treatment, what their wants are and what they’re willing to sustain with regard to frequency of office visits and potential side effects.
Track 4
![]() DR LOVE: How do you approach the issue of HER2-positive metastatic
disease, particularly in light of the fact that now some patients have
received adjuvant trastuzumab?
DR LOVE: How do you approach the issue of HER2-positive metastatic
disease, particularly in light of the fact that now some patients have
received adjuvant trastuzumab?
![]() DR THEODOULOU: For a patient who is trastuzumab naïve, I will administer
trastuzumab. The chemotherapy regimen I use is dependent on prior therapy
and how recently it was administered. Most commonly I administer a weekly
taxane or vinorelbine. The choices, again, are based on the patient’s lifestyle,
goals of treatment, prior therapies and comorbidities.
DR THEODOULOU: For a patient who is trastuzumab naïve, I will administer
trastuzumab. The chemotherapy regimen I use is dependent on prior therapy
and how recently it was administered. Most commonly I administer a weekly
taxane or vinorelbine. The choices, again, are based on the patient’s lifestyle,
goals of treatment, prior therapies and comorbidities.
If the patient experiences disease progression, then I springboard over to lapatinib with capecitabine. For a patient whose disease has recurred within six months of receiving trastuzumab, I administer lapatinib first. Once we reach one year, then I’m willing to consider another trastuzumab regimen.
We have many clinical trials, as most tertiary institutions do today, evaluating all sorts of HER inhibitors, whether it’s HER1, HER2 or intracellular inhibition versus transjunctional membrane inhibition. The trials are exploding.
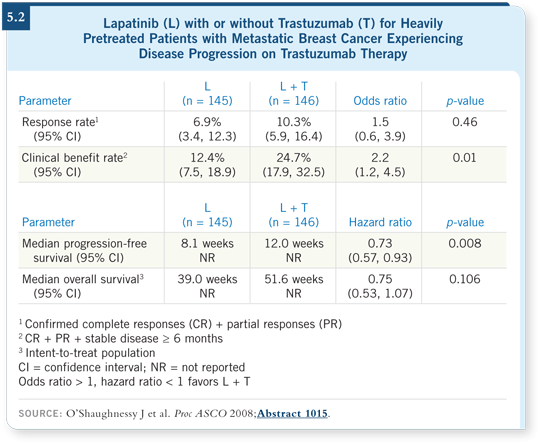
The presentation by Joyce O’Shaughnessy at ASCO this year was interesting. The study evaluated a combination of biologic agents — trastuzumab and lapatinib — in patients who were heavily pretreated, some with up to six prior regimens before entering the study. Patients were randomly assigned to lapatinib alone or in combination with trastuzumab. They reported significant clinical benefit with the combination and approximately a 12 percent benefit with lapatinib alone (O’Shaughnessy 2008; [5.2]). It appears that in the combination, lapatinib potentiated the trastuzumab benefit.
![]() DR LOVE: What are the situations, if any, in which you might use the trastuzumab/lapatinib combination off study right now?
DR LOVE: What are the situations, if any, in which you might use the trastuzumab/lapatinib combination off study right now?
![]() DR THEODOULOU: I would consider it for a patient with excellent cardiac
function for whom a trastuzumab-based regimen had already failed and for
whom lapatinib with capecitabine had already failed and if she were not a
candidate for a clinical trial.
DR THEODOULOU: I would consider it for a patient with excellent cardiac
function for whom a trastuzumab-based regimen had already failed and for
whom lapatinib with capecitabine had already failed and if she were not a
candidate for a clinical trial.
It is encouraging to know that a combination arm that we will be using in the adjuvant setting in the ALTTO trial is safe and feasible.
Track 5
![]() DR LOVE: Could you discuss the presentation at ASCO 2008 that
reported preliminary safety results from your institution of dose-dense AC
followed by weekly paclitaxel with trastuzumab and lapatinib?
DR LOVE: Could you discuss the presentation at ASCO 2008 that
reported preliminary safety results from your institution of dose-dense AC
followed by weekly paclitaxel with trastuzumab and lapatinib?
![]() DR THEODOULOU: Chau Dang’s presentation at ASCO 2008 reported results
of a 100-patient feasibility study of what is to be one of the four arms of the
ALTTO study. On this feasibility study, patients were randomly assigned to
paclitaxel weekly for 12 weeks in combination with lapatinib and trastuzumab
from day one of the paclitaxel after their anthracycline-based treatment (Dang
2008a).
DR THEODOULOU: Chau Dang’s presentation at ASCO 2008 reported results
of a 100-patient feasibility study of what is to be one of the four arms of the
ALTTO study. On this feasibility study, patients were randomly assigned to
paclitaxel weekly for 12 weeks in combination with lapatinib and trastuzumab
from day one of the paclitaxel after their anthracycline-based treatment (Dang
2008a).
The primary endpoint of the trial was cardiac safety — defined as discontinuation of trastuzumab in combination with lapatinib resulting from cardiac death or congestive heart failure. If less than 20 percent of the patients were not able to complete, or if the cardiac toxicity was not any greater than what had been reported in adjuvant trastuzumab trials to date, that would be okay. But our trial was not okay. Patients couldn’t tolerate the regimen. One third of these patients had extensive gastrointestinal side effects with diarrhea. Of the patients, 27 percent had to be pulled off the study, so the study was stopped at 95 patients, and the message of the study was, “This cannot be done” in the doses that were being administered for lapatinib with paclitaxel and trastuzumab.
Patients who then went on to continue paclitaxel with trastuzumab fared well, as did patients who continued lapatinib and trastuzumab. But it was that triplet that got patients into trouble. Obviously that fourth arm will change in the ALTTO trial by way of the dosing of lapatinib.
Track 6
![]() DR LOVE: What do you think about the current data in terms of the use
of lapatinib in a patient with brain metastases?
DR LOVE: What do you think about the current data in terms of the use
of lapatinib in a patient with brain metastases?
![]() DR THEODOULOU: The initial trial by Geyer made everybody “sit up straight
in their seats” when he reported 11 brain relapses in the capecitabine-alone arm
and only four in the capecitabine with lapatinib arm (Geyer 2006; Cameron
2008).
DR THEODOULOU: The initial trial by Geyer made everybody “sit up straight
in their seats” when he reported 11 brain relapses in the capecitabine-alone arm
and only four in the capecitabine with lapatinib arm (Geyer 2006; Cameron
2008).
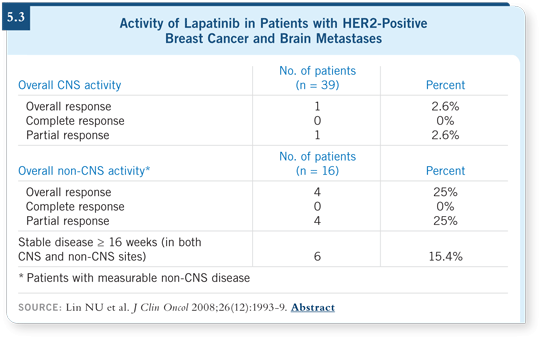
A study at Dana-Farber also evaluated lapatinib in patients with brain metastases. They reported what we’d consider a minor tumor volume reduction — less than 25 percent of the volume that was initially presented as a reduction, with a lot of stable disease (Lin 2008; [5.3]). But a minor response is huge in patients with brain metastases.
Track 7
![]() DR LOVE: What schedule do you use when administering lapatinib/capecitabine off study?
DR LOVE: What schedule do you use when administering lapatinib/capecitabine off study?
![]() DR THEODOULOU: I’m using our one-week-on, one-week-off capecitabine
schedule. I haven’t used the 14-day-on and the seven-day-off schedule for
years.
DR THEODOULOU: I’m using our one-week-on, one-week-off capecitabine
schedule. I haven’t used the 14-day-on and the seven-day-off schedule for
years.
![]() DR LOVE: What are you seeing in terms of side effects and toxicity with that
combination?
DR LOVE: What are you seeing in terms of side effects and toxicity with that
combination?
![]() DR THEODOULOU: The mucositis and the diarrhea seen in the past have been
attenuated markedly by using the biweekly capecitabine regimen. Lapatinib is
interesting because patients do report diarrhea and acneiform rash with it, but
it’s tolerated pretty well. In these cases, I may choose to dose attenuate, but I
am careful not to stop the regimen or dose attenuate both drugs. I’ll play with
the capecitabine dose more than anything else, but it’s clear if a patient reports
diarrhea that it’s usually from the lapatinib.
DR THEODOULOU: The mucositis and the diarrhea seen in the past have been
attenuated markedly by using the biweekly capecitabine regimen. Lapatinib is
interesting because patients do report diarrhea and acneiform rash with it, but
it’s tolerated pretty well. In these cases, I may choose to dose attenuate, but I
am careful not to stop the regimen or dose attenuate both drugs. I’ll play with
the capecitabine dose more than anything else, but it’s clear if a patient reports
diarrhea that it’s usually from the lapatinib.
With regard to the rash, not every patient will develop a rash, but those who do hate it. It’s usually on the face and upper torso in the chest area. Often, it can be treated effectively with topical antibiotics. If not, I reduce the lapatinib by 250 milligrams.
EDITOR
Neil Love, MD
Paul E Goss, MD, PhD
- Select publications
Martine J Piccart-Gebhart,
MD, PhD
- Select publications
TUMOR PANEL CASE DISCUSSION
- Select publications
INTERVIEWS (continued)
Erica L Mayer, MD, MPH
- Select publications
Maria Theodoulou, MD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity