
 |
||||||||

| Tracks 1-20 | ||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1, 4
![]() DR LOVE: You are coauthor of a study presented at San Antonio on trastuzumab-DM1 (T-DM1). Can you comment?
DR LOVE: You are coauthor of a study presented at San Antonio on trastuzumab-DM1 (T-DM1). Can you comment?
![]() DR BURRIS: I was a skeptic about the idea that a cytotoxic agent could be
linked to an antibody, retained with the antibody and then delivered to the
cancer cells. We have tried this a few times through the years without success.
In T-DM1 the cytotoxic agent is mertansine. Maytansine was the parent
compound, and this is a derivative. We knew that the maytansine compounds
were extremely active but too toxic. With T-DM1, we did not see pancytopenia,
hair loss or other classic toxicities associated with maytansine. However,
we did observe transient changes in platelet counts, which were probably an
immunologic effect.
DR BURRIS: I was a skeptic about the idea that a cytotoxic agent could be
linked to an antibody, retained with the antibody and then delivered to the
cancer cells. We have tried this a few times through the years without success.
In T-DM1 the cytotoxic agent is mertansine. Maytansine was the parent
compound, and this is a derivative. We knew that the maytansine compounds
were extremely active but too toxic. With T-DM1, we did not see pancytopenia,
hair loss or other classic toxicities associated with maytansine. However,
we did observe transient changes in platelet counts, which were probably an
immunologic effect.
In the Phase II trial, the median time on prior trastuzumab was approximately 76 weeks, and the response rates to T-DM1 were 30 to 40 percent among patients with previously treated, HER2-positive metastatic disease (Vukelja 2008).
![]() DR LOVE: Where do you see T-DM1 heading?
DR LOVE: Where do you see T-DM1 heading?
![]() DR BURRIS: T-DM1 is moving forward, and I believe that in a year it will
be utilized as second-line therapy. The data from this study are so promising
that a randomized trial (NCT00679341) has recently been initiated comparing
T-DM1 to trastuzumab/docetaxel as first-line therapy for HER2-positive
metastatic disease.
DR BURRIS: T-DM1 is moving forward, and I believe that in a year it will
be utilized as second-line therapy. The data from this study are so promising
that a randomized trial (NCT00679341) has recently been initiated comparing
T-DM1 to trastuzumab/docetaxel as first-line therapy for HER2-positive
metastatic disease.
Track 9
![]() DR LOVE: Would you discuss your group’s study headed by Denise
Yardley evaluating the addition of bevacizumab to three different
docetaxel-containing adjuvant regimens?
DR LOVE: Would you discuss your group’s study headed by Denise
Yardley evaluating the addition of bevacizumab to three different
docetaxel-containing adjuvant regimens?
![]() DR BURRIS: The idea was to take the docetaxel-containing regimens that
were becoming standards — such as TCH (docetaxel/carboplatin/trastuzumab)
— and add the VEGF inhibitor bevacizumab. The goal of our trial
was to provide safety data for the BETH study and other trials.
DR BURRIS: The idea was to take the docetaxel-containing regimens that
were becoming standards — such as TCH (docetaxel/carboplatin/trastuzumab)
— and add the VEGF inhibitor bevacizumab. The goal of our trial
was to provide safety data for the BETH study and other trials.
We presented preliminary results at ASCO 2008 (Hart 2008), and the report
at San Antonio was a follow-up confirmation. We treated 75 patients with
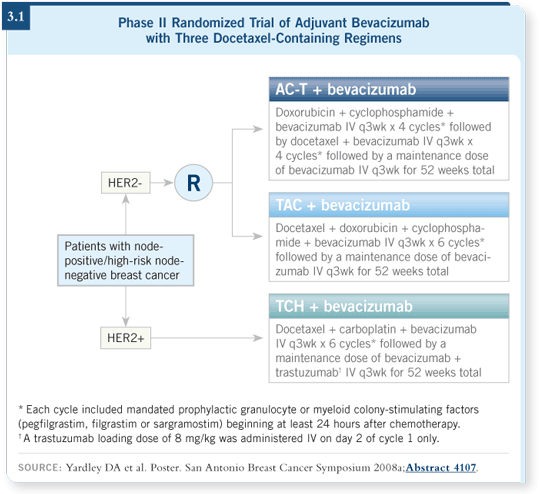
bevacizumab in combination with the following regimens: TCH, AC ![]() docetaxel
or TAC (docetaxel/doxorubicin/cyclophosphamide; Yardley 2008a;
[3.1]). With TCH and bevacizumab, the main concern was cardiac toxicity.
We didn’t have any problems — only two of 75 patients showed declines in
their ejection fractions (Yardley 2008a).
docetaxel
or TAC (docetaxel/doxorubicin/cyclophosphamide; Yardley 2008a;
[3.1]). With TCH and bevacizumab, the main concern was cardiac toxicity.
We didn’t have any problems — only two of 75 patients showed declines in
their ejection fractions (Yardley 2008a).
As we proceed into adjuvant trials with bevacizumab, the results improve with regard to cardiac toxicity because nurses and doctors are becoming internists and are treating hypertension. We no longer have hypertension problems with bevacizumab because we administer ACE inhibitors and diuretics earlier.
Tracks 14-15
![]() DR LOVE: Would you discuss your neoadjuvant study presented at the
2008 SABCS meeting?
DR LOVE: Would you discuss your neoadjuvant study presented at the
2008 SABCS meeting?
![]() DR BURRIS: The neoadjuvant trial involved an aggressive regimen of
gemcitabine in combination with epirubicin and nanoparticle albumin-bound
(nab) paclitaxel administered as a dose-dense, every other-week approach.
DR BURRIS: The neoadjuvant trial involved an aggressive regimen of
gemcitabine in combination with epirubicin and nanoparticle albumin-bound
(nab) paclitaxel administered as a dose-dense, every other-week approach.
Our pathologic complete response (CR) rate in the breast and lymph nodes was 18 percent. These were patients with initially unresectable or difficult to resect tumors (Yardley 2008b). This was an interesting result. We used pegfilgrastim support, and the toxicity was manageable. We also evaluated the patients’ SPARC status, and a trend toward a higher pathologic CR rate was recorded among the patients with SPARC-positive disease (Yardley 2008b).
![]() DR LOVE: What benefits do you believe nab paclitaxel provides?
DR LOVE: What benefits do you believe nab paclitaxel provides?
![]() DR BURRIS: Not having to use steroids is attractive, particularly for patients
with diabetes or other preexisting conditions. The second benefit is being able
to administer the drug without fear of a hypersensitivity reaction. They’re
uncommon, but in a busy clinic such as ours, we see one every few weeks.
DR BURRIS: Not having to use steroids is attractive, particularly for patients
with diabetes or other preexisting conditions. The second benefit is being able
to administer the drug without fear of a hypersensitivity reaction. They’re
uncommon, but in a busy clinic such as ours, we see one every few weeks.
![]() DR LOVE: What about the potential for increased efficacy?
DR LOVE: What about the potential for increased efficacy?
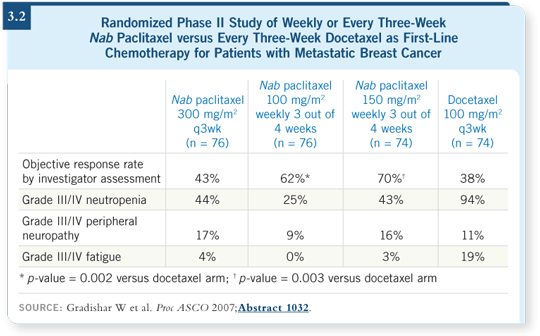
![]() DR BURRIS: In the trial comparing it to paclitaxel in metastatic disease, the
response rate and time to progression were better (Gradishar 2005). In Bill
Gradishar’s follow-up randomized Phase II study, weekly nab paclitaxel appears
to carry a preferential advantage compared to docetaxel (Gradishar 2007; [3.2]).
DR BURRIS: In the trial comparing it to paclitaxel in metastatic disease, the
response rate and time to progression were better (Gradishar 2005). In Bill
Gradishar’s follow-up randomized Phase II study, weekly nab paclitaxel appears
to carry a preferential advantage compared to docetaxel (Gradishar 2007; [3.2]).
EDITOR
Neil Love, MD
Harold J Burstein, MD, PhD
- Select publications
Jack Cuzick, PhD
- Select publications
Howard A Burris III, MD
- Select publications
Mark D Pegram, MD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity